Abstract
We performed real-time myocardial contrast echocardiography on a patient with cardiac amyloidosis and previous normal coronary angiography presenting with atypical chest pain to assess myocardial blood flow reserve (MBFR). Myocardial contrast echocardiography was performed and flash microbubble destruction and replenishment analysis was used to calculate myocardial blood flow. Dipyridamole was used to achieve hyperemia. MBFR was derived from the ratio of peak myocardial blood flow at hyperemia and rest. The results show a marked reduction in MBFR in our patient. Previous reports of luminal obstruction of intramyocardial rather than epicardial vessels by amyloid deposition may be causing microvascular dysfunction.
Amyloid light-chain (AL) amyloidosis has an annual incidence of 6–10 cases per million in the USA with cardiac involvement occurring in 90%.1) The clinical presentation of cardiac amyloidosis (CA) in the form of heart failure due to restrictive infiltrative cardiomyopathy is well described. Less often patients present with myocardial ischemic syndromes which is thought to be due to amyloid obstruction of the intramyocardial coronary arteries.2) Little has been published on the effect of CA on microcirculatory function. Here we demonstrate a reduction of myocardial blood flow reserve (MBFR) in a patient with CA using real-time myocardial contrast echocardiography (MCE).
A 52-year-old lady who presented with breathlessness and mild left ventricular impairment with severe biventricular thickening on echocardiography. There was no past medical history of note and blood pressure assessment was normal. Haematological investigations revealed systemic light chain (AL) amyloidosis. Cardiac magnetic resonance imaging (CMR) showed biatrial and biventricular extensive subendocardial delayed enhancement typical for CA (Fig. 1). Although endomyocardial biopsy was not performed, the diagnosis of CA was based on confirmed plasma-cell dyscrasia with presence of amyloid in bone marrow biopsy, and typical amyloid CMR (CMR carries 92% positive predictive value).3) Additionally, repeat echocardiogram and CMR following chemotherapy showed restoration of left ventricular systolic function and reduction in subendocardial delayed enhancement. The patient had a normal coronary angiogram one year previously for investigation of chest pain. After developing further atypical chest pains she was referred for dipyridamole stress echocardiography to assess for wall motion abnormality, which was negative. We subsequently performed vasodilator MCE to assess myocardial blood flow (MBF) at baseline, MBF at peak stress (MBFs) and MBFR. MBFR is a validated technique in the assessment of microvascular function in the absence of obstructive coronary artery disease first reported by Wei et al.4) in 2001. Real-time MCE was performed according to an established protocol using a commercial ultrasound machine (iE33, Philips Medical Systems, Best, the Netherlands) and SonoVue (Bracco Research SA, Geneva, Switzerland) contrast agent with a rate set between 0.8–1.0 mL/min to maximize image quality with minimal attenuation.5) Images were recorded in the 3 apical views with low-power settings at a mechanical index of 0.1. Flash-impulse imaging at a high mechanical index (1.0) was performed to achieve complete myocardial bubble destruction, after which 10 end-systolic frames were recorded digitally in each view. Imaging was repeated after dipyridamole infusion at 0.56 mg/kg over a 4-minute period. After an interval of 2 minutes, dipyridamole vasodilator images were recorded within 3 to 4 minutes. Excellent image quality was obtained with all 16 left ventricular segments adequately visualized. Quantitative analysis was performed by an investigator who was blinded to the CMR data. Standard commercial software (Qlab version 7.0, Q-Laboratory, Philips Medical Systems, Best, the Netherlands) was used to study regions of interest within the myocardium and quantify myocardial replenishment as previously described.4) Basal segments of the 16-segment left ventricular model were not included in the quantitative analysis.
Background-subtracted plots of peak myocardial contrast intensity (representing myocardial blood volume A, dB) versus pulsing intervals (representing time) were automatically constructed by QLab software to fit the monoexponential function conventional equation: y = A (1 - e-βt)2. From these plots, the slope of the replenishment curve was determined (representing myocardial blood velocity β, dB/s). Per segment, the product of A and β yielded rest MBF and post-dipyridamole MBFs (stress MBF, dB2/s), respectively. The replenishment curves are presented in Fig. 2. MBFR was calculated as the ratio of peak MBF to resting MBF.
The replenishment pictures are shown in Fig. 3. Although steady state perfusion at rest and peak stress appears homogenous throughout the myocardium the rate of replenishment following bubble destruction at rest and peak stress is significantly reduced. The peak myocardial blood velocity β was therefore less than normal healthy studies. Although the peak MBF increased (26.9 dB2/s vs. 43.9 dB2/s) the MBFR was only 1.6 (normal > 2).6)
CA is a rare condition associated with primary light chain (AL) amyloidosis. The impairment in MBF observed in CA has been described in two small case studies previously and is thought to be due to obstruction of the intramyocardial coronary arterial lumen by amyloid deposition.2) In contrast, although the larger epicardial arteries show evidence of amyloidosis in the arterial walls, the lumen of these vessels are almost always unobstructed. Vascular deposition of amyloid, although not as common as interstitial type, is well described in the literature.2)7) The intramyocardial arteries are selectively affected with deposition first beginning in the media and then progressing to the adventitia.1) Eventually, luminal obstruction occurs and in one series was shown to be histologically present in two thirds of patients studied.2) This effect is thought to be the cause of the anginal symptoms described and be responsible for the ischemic histological changes seen in the majority of amyloid hearts. It may also explain the chronically elevated troponin levels observed.2)7)8)
Real time MCE with flash bubble destruction is a well-validated diagnostic tool to quantify MBFR.9) Two previous studies have examined MBF in patients with CA. Al Suwaidi et al.10) undertook coronary angiography in five patients with CA and measured coronary flow reserve using adenosine and then acetylcholine. Although all had normal epicardial arteries, both endothelial independent and endothelial dependent functions were significantly impaired. In a second study, a single case of amyloid underwent MCE with vasodilator stress in which MBF was also impaired.11) This report further demonstrates the use of this technique in understanding CA pathophysiology. In addition we observe that MBFR is lower in our amyloid patient because of peak blood velocity β being lower compared to healthy population data. Overall, microvascular ischaemia resulting from intramyocardial coronary artery obstruction by amyloid deposition can be a probable cause of abnormal MBFR of CA even without epicardial coronary artery disease.
Figures and Tables
Fig. 1
Cardiac MR four chamber view showing biatrial and biventricular subendocardial delayed enhancement in keeping with a diagnosis of cardiac amyloidosis.
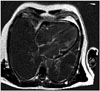
Fig. 2
Replenishment curves following bubble destruction using high amplitude ultrasound pulse at rest (A) and post-dipyridamole (stress) (B). A normal study curve has also been plotted for comparison, taken from previous published data by our group.5) Peak video intensity A is proportional to and therefore representative of blood volume. Gradient β (dB/s) represents peak blood velocity.

Fig. 3
Real-time myocardial contrast echo images showing bubble replenishment at rest and stress with a normal study for comparison. Each number in top left of image represents number of frames post bubble destruction. Notice bubble replenishment is more rapid and homogenous in the normal study compared to our patient both at rest and stress.

References
1. Narang R, Chopra P, Wasir HS. Cardiac amyloidosis presenting as ischemic heart disease. A case report and review of literature. Cardiology. 1993; 82:294–300.
2. Neben-Wittich MA, Wittich CM, Mueller PS, Larson DR, Gertz MA, Edwards WD. Obstructive intramural coronary amyloidosis and myocardial ischemia are common in primary amyloidosis. Am J Med. 2005; 118:1287.
3. Vogelsberg H, Mahrholdt H, Deluigi CC, Yilmaz A, Kispert EM, Greulich S, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008; 51:1022–1030.
4. Wei K, Ragosta M, Thorpe J, Coggins M, Moos S, Kaul S. Noninvasive quantification of coronary blood flow reserve in humans using myocardial contrast echocardiography. Circulation. 2001; 103:2560–2565.
5. Rana O, Byrne CD, Kerr D, Coppini DV, Zouwail S, Senior R, Begley J, Walker JJ, Greaves K. Acute hypoglycemia decreases myocardial blood flow reserve in patients with type 1 diabetes mellitus and in healthy humans. Circulation. 2011; 124:1548–1556.
6. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007; 356:830–840.
7. Mueller PS, Edwards WD, Gertz MA. Symptomatic ischemic heart disease resulting from obstructive intramural coronary amyloidosis. Am J Med. 2000; 109:181–188.
8. Apridonidze T, Steingart RM, Comenzo RL, Hoffman J, Goldsmith Y, Bella JN, Landau H, Liu JE. Clinical and echocardiographic correlates of elevated troponin in amyloid light-chain cardiac amyloidosis. Am J Cardiol. 2012; 110:1180–1184.
9. Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, Nihoyannopoulos P. Contrast echocardiography: evidencebased recommendations by European Association of Echocardiography. Eur J Echocardiogr. 2009; 10:194–212.
10. Al Suwaidi J, Velianou JL, Gertz MA, Cannon RO 3rd, Higano ST, Holmes DR Jr, Lerman A. Systemic amyloidosis presenting with angina pectoris. Ann Intern Med. 1999; 131:838–841.
11. Abdelmoneim SS, Bernier M, Bellavia D, Syed IS, Mankad SV, Chandrasekaran K, Pellikka PA, Mulvagh SL. Myocardial contrast echocardiography in biopsy-proven primary cardiac amyloidosis. Eur J Echocardiogr. 2008; 9:338–341.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download