Abstract
Pneumopericardium is defined by the presence of air in the pericardial cavity. It is a rare entity occurring most commonly after trauma. Pneumopericardium resulting after pericardiocentesis is even rarer. We report a case of 46-year-old man, with end-stage renal disease on chronic hemodialysis and who developed a large circumferential pericardial effusion of 40 mm in diastole with swinging heart and diastolic right atrium collapse requiring pericardiocentesis. Few days after, the patient complained of pleuritic chest pain and echocardiogram revealed several tiny sparkling echogenic spots swirling in the pericardial sac. Computed tomography scans revealed a marked anterior pneumopericardium that was conservatively managed.
Pneumopericardium is defined as the presence of air in the pericardial cavity. It is a rare entity that has been reported to result most commonly after trauma or spontaneously without underlying cause in healthy adult.1) Pneumopericardium resulting after pericardiocentesis is even rarer and has been attributed either to a direct pleuro-pericardial communication or to an air leakage to the pericardial drainage system.2)3)4) Most of the time iatrogenic pneumopericardium requires no specific therapy but in some patients, life-threatening complications, especially pericardial tamponade, can occur and require prompt recognition and adequate managements.3)4) Pneumopericardium is relatively easy to diagnose by chest radiographs which reveal lucent outline separating the pericardium from the heart or more interestingly by echocardiography which demonstrates swirling bubbles sign in pericardial cavity.3)5) We herein discuss a case of iatrogenic pneumopericardium in an adult man complicating therapeutic pericardiocentesis, diagnosed by echocardiography and confirmed by chest computed tomography (CT).
We report a case of 46-year-old man with end-stage renal failure on chronic hemodialysis, who presented to hospital with a chief complaint of progressively exertional worsening dyspnea of 3-months duration. He was diagnosed to have small amount of pericardial effusion 6 months ago. On admission his physical exam revealed stable hemodynamics (blood pressure, 142/ 85 mm Hg; pulse rate, 72 bpm; respiratory rate, 24 per minute; body temperature, 37.2°C) with muffled heart sounds on cardiac auscultation. Electrocardiogram showed sinus rhythm with low QRS complex voltage in frontal plane leads. Transthoracic echocardiography (TTE) was done and demonstrated a large circumferential pericardial effusion with swinging heart and diastolic right atrium collapse without significant respiratory variations of the mitral inflow or aortic flow (Fig. 1 and 2, Supplementary movie 1 and 2). Drainage of pericardial fluid by percutaneous pericardiocentesis using the Seldinger technique via subxiphoid approach with echo-guidance was performed. Intrapericardial catheter was secured to the skin and attached to a closed drainage system under negative pressure using a vacuum container. Over 1600 mL of serosanguineous fluid was drained over the following 48 hours. The indwelling catheter was removed after. Pericardial fluid was a lymphocyte dominant exudate with normal white blood cell count. Cytological exam was negative for malignant cells, cultures and smear for acid-fast bacilli and other organisms were negative too. Blood urea nitrogen levels were elevated and we concluded to hemorrhagic uremic chronic pericardial effusion. Ten days after, the patient complained of occasional pleuritic chest pain with no other symptoms. Physical examination revealed stable vital signs. Control TTE showed normal left ventricular ejection fraction with normal aortic flow, no measurable tricuspid valve regurgitation and scanty pericardial effusion with several tiny sparkling echogenic spots swirling in the pericardial sac evoking micro air bubbles (Fig. 3, Supplementary movie 3 and 4), and a partial disappearance of the shape of cardiac chambers in late systole with a band of echoes within (Fig. 4, Supplementary movie 4). No echocardiographic features of tamponade were found and a pneumopericardium was suspected according to these findings. Chest CT scan revealed a marked hemopneumopericardium with an anterior extent (Fig. 5). Owing to a stable hemodynamic status with no clinical or echocardiographycal signs of tension pneumopericardium, the patient was closely monitored and managed conservatively under watchful surveillance. Spontaneous healing occurred the following days.
Pneumopericardium is defined as an accumulation of air-fluid level in the pericardial cavity. It is a rare but can be life-threatening entity that has been reported to result after a multitude of causes such as penetrating or blunt chest trauma, pericardium infections, iatrogenic and invasive procedures, abnormal communications such as fistula between the pericardium and hallow organs, or spontaneously without any underlying cause in healthy adult.1)2)3)4)6)7)8)9)10)
Iatrogenic pneumopericardium occurring after therapeutic pericardiocentesis is even rarer and has been reported in few cases of literature.2)3)5)6) It has been attributed either to a direct pleuro- pericardial communication or to an air leakage to the pericardial drainage system.2)3)4) Pneumopericardium after surgical pericardiotomy has been reported to our knowledge in one only case in the literature and was attributed too, to an air leakage to pericardial drainage tubing that was inadvertently left open to room air.11)
Clinical manifestations are variable and unspecific (pain, dyspnea, palpitations ...), moreover patients are most commonly asymptomatic what makes a real challenge for early detection of the disease before the current hemodynamic situation of the patient worsens.4)6) In symptomatic patients, symptoms are tributary of the abundance, the extent of the pneumopericardium and the underlying etiology.4) Cardiac auscultation can reveal pathognomic signs such as the mill wheel murmur "bruit de moulin" heard as a succession splash and traducing shaking movement of the heart within pericardial cavity.4) The pneumopericardium can be relatively easy diagnosed by chest radiographs which reveal air as a radiolucent rim separating the pericardium from the heart, called "Continuous diaphragm sign".3)4)12) TTE findings can show two pathognomic signs: "The air gap sign" traducing a cyclic disappearance of the cardiac shape during systole coinciding with a cycling appearance of air within the pericardium during this phase as the volume of cardiac cavities decreases13)14) and "The swirling bubbles sign" representing the presence of an air-fluid interface with continuous churning movements in pericardial cavity due to heart activity and which is revealed in echocardiography by several tiny bright echogenic spots in the pericardial sac evoking micro air bubbles.3)5)12)15) Chest CT can easily confirm the diagnosis and is the mainstay of diagnosis of pneumopericardium in obscure cases. It offers further information concerning mechanisms and associate lesions.7)
The clinical course of pneumopericardium remains highly variable and the most redoubtable complication is tension pneumopericardium that can be fatal. It is clear that the abundance and especially the speed of constitution of pneumopericardium are the main determinant of clinical severity that will guide with the underlying etiology the therapeutic strategy.4)5)6)16) Usually pneumopericardium resolves spontaneously.2)3)16)17) In the presence of acute hemodynamic imbalance suggesting cardiac tamponade, an emergent pericardiocentesis with hemodynamic monitoring are required to promptly restore hemodynamic stability, followed by pericardial fenestration and drainage.4)10) Surgery is preferred in cases of direct breach between the pericardial cavity and hollow organs apart from lungs to close channel between pericardium and air.5)6)17) In the case of iatrogenic pneumopericardium following pericardiocentesis for pericardial effusion, a re-pericardiocentesis is indicated if hemodynamic conditions are unstable. Watchful waiting for spontaneous absorption of air under a close monitoring is indicated in the absence of acute and life threatening tension pneumopericardium.3)4)17)
In our case report the plausible hypothesis for pneumopericardium is a leaky drainage system. There are no evident arguments to our knowledge in the literature that can prove the inherence of such complication to renal failure or chronic hemodialysis; however the distribution of fibrosis and inflammation in uremic pericarditis can lead to constitution of serosanguineous effusion localized between adherent fibrous bands18)19) what may favor such complication while pericardiocentesis procedure. We highlight too the importance of TTE in establishing the diagnosis of pneumopericardium though the absence of typical clinic signs and symptoms in our case. In fact, a good knowledge of typical signs of pneumopericardium in echocardiography such as "The air gap sign" or "The swirling bubbles sign" as in our case report which gathers the two signs, facts that constitute his originality, led us to evoke the diagnosis that was confirmed after by CT scan. Finally we remind that a spontaneous healing can occur in few days and pneumopericardium can resolves spontaneously as with our patient, but it requires a close monitoring and watchful surveillance to exclude a cardiac tamponade that can happens in any moment and should be urgently ruled out.
Figures and Tables
Fig. 1
Two dimensional echocardiography. Parasternal long-axis view showing a large circumferential pericardial effusion of 40 mm in diastole. LV: left ventricle, PE: pericardial effusion.
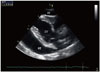
Fig. 2
Pulsed wave Doppler mode showing no significant respiratory variations of the mitral inflow (A) and aortic flow (B).
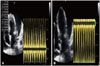
Fig. 3
Two dimensional echocardiography. Parasternal short-axis view showing several tiny sparkling echogenic spots swirling in the pericardial sac evoking micro air bubbles (open arrows). PE: pericardial effusion, RV: right ventricle, Ao: aorta, PA: pulmonary artery.
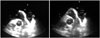
References
1. Lee YJ, Jin SW, Jang SH, Jang YS, Lee EK, Kim YJ, Lee MY, Park JC, Rho TH, Kim JH, Hong SJ, Choi KB. A case of spontaneous pneumomediastinum and pneumopericardium in a young adult. Korean J Intern Med. 2001; 16:205–209.
2. Mullens W, Dupont M, De Raedt H. Pneumopericardium after pericardiocentesis. Int J Cardiol. 2007; 118:e57.
3. Choi WH, Hwang YM, Park MY, Lee SJ, Lee HY, Kim SW, Jun BY, Min JS, Shin WS, Lee JM, Koh YS, Jeon HK, Chung WS, Seung KB. Pneumopericardium as a complication of pericardiocentesis. Korean Circ J. 2011; 41:280–282.
4. Brander L, Ramsay D, Dreier D, Peter M, Graeni R. Continuous left hemidiaphragm sign revisited: a case of spontaneous pneumopericardium and literature review. Heart. 2002; 88:e5.
5. Yuce M, Sari I, Davutoglu V, Ozer O, Usalan C. Bubbles around the heart: pneumopericardium 10 days after pericardiocentesis. Echocardiography. 2010; 27:E115–E116.
6. Lee SH, Kim WH, Lee SR, Rhee KS, Chae JK, Ko JK. Cardiac tamponade by iatrogenic pneumopericardium. J Cardiovasc Ultrasound. 2008; 16:26–28.
7. Rashid MA, Wikström T, Ortenwall P. Pneumopericardium and pneumoperitoneum after penetrating chest injury. Eur J Surg. 1999; 165:278–279.
8. Müller AM, Betz MJ, Kromeier J, Ghanem NA, Geibel A, Imdahl A, Frydrychowicz AP. Images in cardiovascular medicine. Acute pneumopericardium due to intestino-pericardial fistula. Circulation. 2006; 114:e7–e9.
9. Shackelford RT. Hydropneumopericardium: report of a case with a summary of the literature. JAMA. 1931; 96:187–191.
10. Cummings RG, Wesly RL, Adams DH, Lowe JE. Pneumopericardium resulting in cardiac tamponade. Ann Thorac Surg. 1984; 37:511–518.
11. Marijon E, Jani D, Aubert S. Unusual chest radiograph after surgical pericardiotomy. Tex Heart Inst J. 2007; 34:256–257.
12. Bejvan SM, Godwin JD. Pneumomediastinum: old signs and new signs. AJR Am J Roentgenol. 1996; 166:1041–1048.
13. Reid CL, Chandraratna AN, Kawanishi D, Bezdek WD, Schatz R, Nanna M, Rahimtoola SH. Echocardiographic detection of pneumomediastinum and pneumopericardium: the air gap sign. J Am Coll Cardiol. 1983; 1:916–921.
14. Kerut EK, Hannawalt C, Everson CT, Nanda NC. The air gap sign. Echocardiography. 2014; 31:400–401.
15. Antonini-Canterin F, Nicolosi GL, Mascitelli L, Zanuttini D. Direct demonstration of an air-fluid interface by two-dimensional echocardiography: a new diagnostic sign of hydropneumopericardium. J Am Soc Echocardiogr. 1996; 9:187–189.
16. Uluçam MZ. An extremely rare combination: pneumopericardium, pneumoperitoneum, and subcutanous emphysema-a case report. Cardiol Ther. 2013; 2:103–110.
17. Ozerkan F, Bilgin M, Oktem MS, Alkan MB. [Pneumopericardium after pericardiocentesis: a case report]. Turk Kardiyol Dern Ars. 2011; 39:697–700.
18. Wacker W, Merrill JP. Uremic pericarditis in acute and chronic renal failure. J Am Med Assoc. 1954; 156:764–765.
19. Rostand SG, Rutsky EA. Pericarditis in end-stage renal disease. Cardiol Clin. 1990; 8:701–707.
Supplementary movie legends
Movie 1
Two dimensional echocardiography. Parasternal long-axis view showing a marked circumferential pericardial effusion with swinging heart.
Movie 2
Two dimensional echocardiography. Apical 4-chamber view showing large circumferential pericardial effusion with swinging heart and diastolic right atrium collapse.
Movie 3
Two dimensional echocardiography. Parasternal short-axis view (trans-aortic) showing several tiny sparkling echogenic spots swirling in the pericardial sac evoking micro air bubbles.
Movie 4
Two dimensional echocardiography. Parasternal short-axis view (mid-ventricular) showing several tiny sparkling echogenic spots swirling in the pericardial sac evoking micro air bubbles. We note a partial disappearance of the shape of cardiac chambers in late systole with a band of echoes within traducing the air gap sign.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download