Abstract
Klippel-Trenaunay syndrome is a rare congenital mesodermal abnormality characterized by varicose veins, cutaneous hemangiomas, soft tissue and bony hypertrophy of limb. Potential complications such as deep venous thrombosis and pulmonary thromboembolism have not been reported in Korea to date. We demonstrate the case of a 48-year-old woman with Klippel-Trenaunay syndrome with extensive varicose veins on right lower limb, hypertrophy of left big toe and basilar artery tip aneurysm, complicated with acute submassive pulmonary thromboembolism treated successfully with intravenous thrombolytic therapy.
The Klippel-Trenaunay syndrome is a rare mesodermal abnormality characterized by a triad of vascular nevus, varicose veins, and soft tissue and bony hypertrophy of limb.1) Klippel-Trenaunay syndrome can be diagnosed on the basis of any two of these three features.2) Clinical presentation of Klippel-Trenaunay syndrome has wide spectrum from asymptomatic state to potentially life-threatening complications, such as hypercoagulability, deep vein thrombosis and pulmonary thromboembolism (PTE).1) The hypercoagulability may be attributed to stagnation of blood within the disordered, enlarged venous blood vessels, which can lead to the continuous formation of thrombi, resulting in recurrent PTE.1) Venous thromboembolism has been reported to occur in 8-22% of Klippel-Trenaunay syndrome patient population.3) Over 30 cases on Klippel-Trenaunay syndrome-associated PTE have been reported.3)45)6)7)8) Also, compromised vascular integrity increases risk of thrombus formation of the cerebral aneurysm and stroke in patients with Klippel-Trenaunay syndrome.9)
Here, we report a rare case of Klippel-Trenaunay syndrome with unruptured basilar tip aneurysm, complicated by acute submassive PTE treated successfully with intravenous thrombolytic therapy.
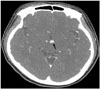
A 48-year-old female presented to the emergency department in our hospital with complaints of severe dyspnea (New York Heart Association III-VI Functional Class) and anterior chest discomfort of two days duration. She had a history of hypertension and took amlodipine besylate 5 mg and atenolol 50 mg daily for antihypertensive medication. Twenty days prior to visit, she had complained of visual impairment and had been diagnosed with compressive optic neuropathy associated with unruptured basilar tip small aneurysm with a diameter of 6 mm detected by enhanced computed tomography (CT) scan of the brain (Fig. 1). She had refused further management for the aneurysm.
On this arrival to emergency department, the patient's blood pressure was 147/94 mm Hg, heart rate was 70 beats/min, respiratory rate was 28 breaths/min, and temperature was 36.4℃. The room air oxygen saturation was 88%. Physical examination revealed large varicose veins on the lateral aspect of right lower limb and hypertrophy of left big toe (Fig. 2). She reported that these varicose veins had been first noticed in early infancy. A diagnosis of Klippel-Trenaunay syndrome was made on the basis of the history and physical examination result. The jugular veins were distended. The grade 2 systolic murmur was audible over the pulmonic area. Chest radiography showed cardiomegaly and enlargement of the pulmonary trunk. An electrocardiogram demonstrated diffuse T wave inversions in the precordial leads with S1Q3T3 pattern (Fig. 3). Laboratory investigations revealed D-dimer level 8.94 µg/mL (reference range: 0-0.5 µg/mL); N-terminal prohormone of brain natriuretic peptide, 7376 pg/mL (0-125 pg/mL); troponin I, 0.12 ng/mL (0-1.0 ng/mL); creatine kinase-myocardial band, 2.0 ng/mL (0-4.9 ng/mL); myoglobin, 23.9 ng/mL (0-107 ng/mL); and high-sensitivity C-reactive protein (CRP), 15.4 mg/L (0-8.0 mg/L). Arterial blood gas analysis at an inspired oxygen fraction of 0.5 results were as follows: pH 7.47; partial pressure of CO2, 36.0 mm Hg; partial pressure of O2, 55.2 mm Hg; plasma bicarbonate concentration, 25.6 mmol/L; and O2 saturation, 89.7%. Bedside transthoracic echocardiography (TTE) revealed flattening of the interventricular septum [D-shaped left ventricle (LV)] and severely dilated right ventricle (RV) with poor systolic function (tricuspid annular plane systolic excursion = 8 mm & RV fractional area change = 24.1%) and severe pulmonary hypertension (estimated pulmonary artery systolic pressure = 112 mm Hg). The RV apex was hyperkinetic and the free wall segment was akinetic, a finding consistent with McConnell's sign (Fig. 4). Acute PTE was highly suspected and enhanced CT scan of the chest confirmed thromboemboli of the both main pulmonary artery branches (Fig. 5). With a diagnosis of acute submassive PTE with intermediate-high risk category in Klippel-Trenaunay syndrome, she was admitted in intensive care unit for close monitoring and detection of haemodynamic decompensation and anticoagulation with low molecular weight heparin (enoxaparin) was immediately administered. One day after admission, she became progressively more tachypneic, hypoxemic and systolic blood pressure (SBP) dropped to 90 mm Hg from 140 mm Hg despite of heparin therapy. The vital signs were the following: blood pressure was 90/60 mm Hg, heart rate was 80 beats/min, respiratory rate was 31 breaths/min. Oxygen saturation was 88% at an inspired oxygen fraction of 0.5. Although she had an unruptured cerebral aneurysm, rescue reperfusion therapy was determined to be performed and she received intravenous thrombolytic therapy with tissue plasminogen activator (tenecteplase), which brought rapid improvement of oxygenation and shortness of breath and normalization of blood pressure. On the fourth day after thrombolysis, repeated TTE showed significant improvement of the RV function (Fig. 4). Anticoagulant with low molecular weight heparin and oral anticoagulant were continued. Venous Doppler studies and enhanced CT venography of the lower extremity performed on the third day after thrombolysis did not reveal evidence of deep venous thrombosis. Additional investigation of inherited thrombophilias did not reveal underlying prothrombotic abnormalities of the coagulation system (antithrombin III, protein C and S, antibodies for Lupus anticoagulants and antiphospholipid). She continued to improve clinically and was discharged home on oral warfarin therapy after 15-day hospital course of treatment. Follow-up enhanced CT scan of the chest obtained 2 month after discharge revealed complete resolution of thrombi of pulmonary arteries (Fig. 5). A follow-up TTE obtained 2 years after discharge demonstrated normal dimension and systolic function of RV and disappearance of the D-shaped LV with complete resolution of McConnell's sign and mild pulmonary hypertension (estimated pulmonary systolic pressure = 50 mm Hg) (Fig. 4). Considering high risk of recurrent PTE, she continued indefinite oral anticoagulation with compression stockings and was asymptomatic during the 3-year follow-up after that episode without any evidence of recurrent PTE or bleeding complications.
Here we report a rare case of Klippel-Trenaunay syndrome with classic triads of vascular nevus, varicose veins and soft tissue hypertrophy. The patient developed acute submassive PTE and was treated successfully with thrombolytic and anticoagulation therapy. Although a few cases of Klippel-Trenaunay syndrome complicated with pseudo-Kaposi's sarcoma,10) bladder hemangioma,11) sigmoid varix,12) lymphangioma circumscriptum13) have reported in Korea, case of Klippel-Trenaunay syndrome complicated with acute thromboembolism has not been reported to date and to the best of our knowledge, this is the first reported case in Korea. Peculiarly, intracranial aneurysm is very rare in Klippel-Trenaunay syndrome with only several cases published to date.9)
This case illustrates the importance of careful history taking and detailed clinical examination for clue to the underlying cause and the diagnosis of PTE which is often incorrectly diagnosed leading to high morbidity and mortality. Although varicose veins and soft tissue hypertrophy are very bizarre findings, these can be missed if careful history and physical examination are not taken.
The venous stasis in vascular malformations of lower limbs in Klippel-Trenaunay syndrome increases the chance of thrombus formation and PTE.1)4) Due to the continuous formation of thrombi, recurrent thromboembolic complications are common in Klippel-Trenaunay syndrome patients and lifelong anticoagulation therapy should be considered in the case of a first deep vein thrombosis or PTE.1)5)
This case demonstrated echocardiographic evidence of RV dysfunction, that is, submassive PTE. The decision to administer a thrombolytic agent in addition to heparin anticoagulation in patients with submassive PTE requires individualized assessment of the balance of benefits versus risk.14) Current guidelines recommend risk-adjusted management strategies in acute PTE and anticoagulation is initially recommended in patients with intermediate-high risk category (right ventricular dysfunction detected on echocardiography and elevated N-terminal prohormone of brain natriuretic peptide level as our case) and close monitoring is recommended in these cases to permit early detection of hemodynamic decompensation and the need for initiation of rescue reperfusion therapy.15) The definition of hemodynamic decompensation is 1) need for CPR, 2) SBP < 90 mm Hg for ≥ 15 minutes, 3) drop in SBP by ≥ 40 mm Hg for ≥ 15 minutes with findings of end organ hypoperfusion (as our case), or 4) need for catecholamines to maintain organ perfusion and SBP > 90 mm Hg, including dopamine infused at > 5 mcg/kg/min.16) Therefore, we thought that our case became hemodynamically decompensated and she needed initiation of rescue thrombolytic therapy. Although this case had unruptured basilar tip aneurysms, there is no evidence of an increased risk of intracranial hemorrhage in patients with pre-existing unruptured cerebral aneurysms who are given thrombolytic therapy.17)18) Thrombolytic therapy was safely administered to our patient, which demonstrated significant clinical improvement without any major bleeding events.
Although the patient was regularly followed-up with oral anticoagulation (in optimal international normalized ratio range) and remained asymptomatic after discharge, mild pulmonary hypertension still persisted on follow-up echocardiogram performed two years after thrombolytic therapy. The possibility of chronic thromboembolic pulmonary hypertension (CTEPH), a rare long-term complication after PTE, must be considered for the cause of pulmonary hypertension. The reported cumulative incidence of CTEPH was 0.1-9.1% within the first two years after a symptomatic PTE event.15) Recurrent thromboembolic complications are also common in Klippel-Trenaunay syndrome patients despite anticoagulation.1) Ventilation-perfusion lung scanning may be used to differentiate CTEPH from other causes of pulmonary hypertension.15) Chest CT scan is used widely for diagnosis of CTEPH.15)
In conclusion, the present case demonstrated a rare and first reported case of Klippel-Trenaunay syndrome with unruptured intracranial aneurysm complicated with acute submassive PTE treated successfully with thrombolytic and anticoagulation therapy. Physicians should be aware of this potentially life-threatening existence and pay particular attention to the physical findings to make the correct diagnosis.
Figures and Tables
Fig. 2
Extensive varicose veins and hypertrophy (arrow) in right lower limb (A) and a hypertrophy (arrow) of soft tissue in left big toe (B).
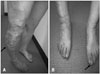
Fig. 3
The 12 leads electrocardiogram demonstrated diffuse T wave inversions in the precordial leads (white arrows) with S1Q3T3 pattern (black arrows).

Fig. 4
Two dimensional TTE. A: Apical-four-chamber view on TTE performed on admission showed marked right ventricular dilatation (solid arrow) with RV/LV ratio > 1. B: Apical-four-chamber view on TTE performed on admission showing hyperkinetic RV apex with akinetic free wall segment (broken arrows) (McConnell's sign). C: Continuous wave Doppler echocardiographic study performed on admission. Pulmonary artery systolic pressure (112 mm Hg) was calculated using maximum velocity of the tricuspid regurgitation jet (Vmax = 4.8 m/sec) and estimated right atrial pressure (20 mm Hg). D: Parasternal short axis view on TTE performed on admission showing flattening of the interventricular septum (D-shaped LV) (solid arrows). E: Repeated TTE performed four days after thrombolysis showed significant reduction of right ventricular cavity (sold arrow) with improved systolic function. F: Apical-four-chamber view on transthoracic echocardiogram performed two years after thrombolysis showing complete resolution (broken arrows) of McConnell's sign. G: Continuous wave Doppler echocardiographic study performed four days after thrombolysis. Pulmonary artery systolic pressure (80 mm Hg) was calculated using maximum velocity of the tricuspid regurgitation jet (Vmax = 4.1 m/sec) and estimated right atrial pressure (10 mm Hg). H: Parasternal short axis view on transthoracic echocardiogram performed two years after thrombolysis showing normalization of RV size and disappearance of the D-shaped LV. LV: left ventricle, RV: right ventricle, TTE: transthoracic echocardiography.
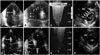
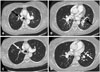
Fig. 5
The computed tomography (CT) scan of the chest performed on admission revealed thromboemboli (black arrows) of the main branches of the right (A) and left (B) pulmonary arteries. Repeated CT scan performed two months after thrombolysis showed complete resolution of thromboemboli (white arrows) of the main branches of the right (C) and left (D) pulmonary arteries.
References
1. Huiras EE, Barnes CJ, Eichenfield LF, Pelech AN, Drolet BA. Pulmonary thromboembolism associated with Klippel-Trenaunay syndrome. Pediatrics. 2005; 116:e596–e600.
2. Jacob AG, Driscoll DJ, Shaughnessy WJ, Stanson AW, Clay RP, Gloviczki P. Klippel-Trénaunay syndrome: spectrum and management. Mayo Clin Proc. 1998; 73:28–36.
3. Baskerville PA, Ackroyd JS, Lea Thomas M, Browse NL. The Klippel-Trenaunay syndrome: clinical, radiological and haemodynamic features and management. Br J Surg. 1985; 72:232–236.
4. Mazoyer E, Enjolras O, Laurian C, Houdart E, Drouet L. Coagulation abnormalities associated with extensive venous malformations of the limbs: differentiation from Kasabach-Merritt syndrome. Clin Lab Haematol. 2002; 24:243–251.
5. Oduber CE, van Beers EJ, Bresser P, van der Horst CM, Meijers JC, Gerdes VE. Venous thromboembolism and prothrombotic parameters in Klippel-Trenaunay syndrome. Neth J Med. 2013; 71:246–252.
6. Muluk SC, Ginns LC, Semigran MJ, Kaufman JA, Gertler JP. Klippel-Trénaunay syndrome with multiple pulmonary emboli--an unusual cause of progressive pulmonary dysfunction. J Vasc Surg. 1995; 21:686–690.
7. Ndzengue A, Rafal RB, Balmir S, Rai DB, Jaffe EA. Klippel-Trenaunay syndrome: an often overlooked risk factor for venous thromboembolic disease. Int J Angiol. 2012; 21:233–236.
8. Yamada T, Ohba T, Yamamoto T, Kimata N, Inami T, Munakata R, Murakami D, Maruyama M, Takano M, Ibuki C, Hata N, Seino Y, Mizuno K. A 17-year-old girl with Klippel-Weber syndrome complicated with a pulmonary thromboembolism and RV thrombus. Intern Med. 2013; 52:1337–1340.
9. Star A, Fuller CE, Landas SK. Intracranial aneurysms in Klippel-Trenaunay/Weber syndromes: case report. Neurosurgery. 2010; 66:E1027–E1028. discussion E1028
10. Kim HJ, Kim SC. A case of pseudo-Kaposi's sarcoma associated with Klippel-Trenaunay syndrome. Korean J Dermatol. 2006; 44:450–453.
11. Lee YK, Kim JK, Cho KS. Bladder hemangioma associated with Klippel-Trenaunay syndrome: case report. J Korean Radiol Soc. 2003; 48:271–274.
12. Chung YW, Han DS, Paik CH, Park YK, Kim JP, Lee HL, Kim JB, Sohn JH, Hahm JS, Park HK. A case of Klippel-Trenaunay-Weber syndrome with sigmoid varices. Korean J Gastrointest Endosc. 2003; 27:21–25.
13. Kim JY, Lee JB, Lee SC, Won YH. A case of Klippel-Trenaunay syndrome with lymphangioma circumscriptum. Korean J Dermatol. 2000; 38:1522–1526.
14. Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. American Heart Association Council on Peripheral Vascular Disease. American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011; 123:1788–1830.
15. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M. Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014; 35:3033–3069. 3033a–3069k.
16. Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galiè N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, Lang IM, Lankeit M, Meneveau N, Pacouret G, Palazzini M, Petris A, Pruszczyk P, Rugolotto M, Salvi A, Schellong S, Sebbane M, Sobkowicz B, Stefanovic BS, Thiele H, Torbicki A, Verschuren F, Konstantinides SV. PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014; 370:1402–1411.
17. Sumner CJ, Golden JA, Hemphill JC 3rd. Should thrombolysis be contraindicated in patients with cerebral arteriovenous malformations. Crit Care Med. 2002; 30:2359–2362.
18. Edwards NJ, Kamel H, Josephson SA. The safety of intravenous thrombolysis for ischemic stroke in patients with pre-existing cerebral aneurysms: a case series and review of the literature. Stroke. 2012; 43:412–416.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download