Abstract
We describe a case of Takotsubo cardiomyopathy in an elderly woman after status epilepticus. In an emergency echocardiography, not only left ventricular apical ballooning but also right ventricular apical hypokinesia was observed. After a medical management, the patient's condition was improved and a follow-up echocardiography showed substantial recovery of left and right ventricular apical ballooning.
Neuro-cardiogenic interaction has been known for many years.1)2) An excessive release of catecholamines, known as a trigger of Takotsubo cardiomyopathy (TC), is developed when sympathetic nervous system is hyperactivated in stressful conditions including cerebral seizure. Cerebral seizure has been reported as a cause of TC internationally, and cardiac complications are one of the main causes of mortality in epilepsy.3)4)
Biventricular TC is associated with more hemodynamic instability than isolated left ventricular TC. Therefore, in that case, medical treatment should be more invasive and the course of hospitalization is longer.5)
But, there has been no case report describing biventricular TC after cerebral seizure in Korea. Here, we report a case of a patient who presented with initial echocardiography showing biventricular apical ballooning after status epilepticus.
An 83-year-old female presented to our emergency room with 2 episodes of 10 minute-duration of generalized tonic-clonic seizures at home and in the ambulance.
Her height was 155 cm, body weight was 42.2 kg. Blood pressure (BP) was 160/100 mm Hg, pulse rate was 105/min, respiration rate was 22/min, and body temperature was 37.0℃. Peripheral oxygen saturation was 96% with oxygen flow of 6 L/min via facial mask.
She had an epilepsy 7 years prior to this attack after cerebral hemorrhage on left parietal and right temporal lobe. She was on donepezil 10 mg, methylphenidate 10 mg, choline alfoscerate 400 mg, acetyl L-carnitine 500 mg, trifrusal 300 mg because of her vascular dementia. She did not have hypertension, diabetes mellitus or dyslipidemia. She was independent in her activities of daily living and had never complained about chest pain or dyspnea before the admission.
When she was admitted, there was another 5-minute duration of generalized tonic-clonic seizure with conjugate deviation of the eyes to the right. Status epilepticus was terminated after intravenous lorazepam 4 mg injection and phenytoin 750 mg infusion. But BP was dropped from 160/100 mm Hg to 70/40 mm Hg and her consciousness was impaired. Central venous catheter was inserted and hemodynamic support was done for a cardiogenic shock.
Chest X-ray showed cardiomegaly and patchy increased opacities in right upper and lower lobes (Fig. 1). Arterial blood gas analysis showed a metabolic acidosis (pH: 7.24, PaO2: 70 mm Hg, PaCO2: 39 mm Hg, HCO3-: 16.7 mmol/L). A diffusion-weighted magnetic resonance brain imaging showed localized encephalomalacic lesions in left parietal lobe and right temporal pole region with peripheral old blood product deposition.
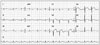
Division of cardiology was called for suspecting ST elevation myocardial infarction (STEMI) since an electrocardiogram (ECG) showed precordial V2-4 ST segment elevation and she was found to have elevated cardiac enzymes on serial measurements (Troponin I levels 0.62 µg/L and 4.87 µg/L respectively) (Fig. 2). There was no evidence of pericarditis or pheochromocytoma from her history, clinical symptoms, laboratory findings and echocardiographical features.
An emergency transthoracic echocardiography (TTE) showed an apical ballooning of the left ventricle (LV) with severe systolic dysfunction (LV ejection fraction = 23% by Simpsons methods) and focal hypokinesia of right ventricular (RV) apex with decreased RV systolic function. Fractional area change (FAC), [(RV end diastolic area - RV end systolic area) / RV end diastolic area × 100] was 22 %, tricuspid annular plane systolic excursion was 20 mm. These impairments extended beyond a coronary artery distribution (Fig. 3).
Despite TTE finding was accordant with TC, emergency coronary angiography was done since we suspected STEMI. There was no significant stenosis in left anterior descending artery, left circumflex artery and right coronary artery. But we found a focal significant stenosis in ramus intermedius artery (Fig. 4). However, hypokinesia of the mid to apical LV and RV from TTE were discordant with coronary artery lesion.
She had no further epileptic seizures during hospitalization with sodium valproate 300 mg every 12 hours and initial metabolic acidosis was resolved. After supportive care with standard heart failure therapy (aspirin, ß-blocker, angiotensin converting enzyme inhibitor, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, diuretics and anti-convulsant), her clinical condition was getting better and moved from intensive care unit to a general ward.
When patient was hospitalized for 10 days, follow-up echocardiography was taken. In TTE, mid to apical wall motion of LV was almost improved except for apico-anterior wall and apical RV wall motion abnormality was not observed any more (Fig. 5). RV FAC was improved from 22% to 44%. The patient was discharged 14 days after admission on foot.
TC, also known as left apical ballooning syndrome or stress-induced cardiomyopathy, is named for ventricle which seems similar in appearance to a Japanese octopus trap on ventriculography scan.6) TC is becoming well recognized as a cause of acute, reversible, and transient LV systolic dysfunction.
In some cases, it would not be simple to make difference between acute coronary syndrome and TC since ECG abnormalities, elevated cardiac enzyme and symptoms mimicking STEMI are common findings in TC. ST-segment elevation is the most common ECG abnormality, reported in about 82% of patients, and T-wave inversion in 64%.7) Generally in TC, there is no identifiable coronary culprit lesion explaining the wall-motion abnormality.
But, as in our case, several cases of TC with coronary atherosclerotic lesion were reported.8) Even though coronary atherosclerotic lesion was present, it was discordant with echocardiographic findings and affected regions were reversibly recovered without primary coronary intervention.9) In our case, there was a significant stenosis in ramus intermedius artery, however, wall motion abnormality was seen in mid to apical LV and apical RV in TTE. Those regional wall motion abnormalities extend beyond a single epicardial vascular distribution and discordant with coronary atherosclerotic lesion. As a result of this, we could diagnose TC.
In previous studies, patients with status epilepticus have high level of plasma catecholamine. When seizure begins, it rises quickly within 30 min and decreases within few hours.10)11) A similar catecholamine release was observed in a case of TC associated with epilepsy.12) Therefore, we can assume that seizure may trigger a condition inducing TC.
In TC associated with epilepsy, complications were frequent and severe: heart failure, apical thrombus, cardiogenic shock, and left ventricular rupture were found in previous studies.13) Although there is no hard data, it could be hypothesized that TC may be related to sudden unexpected death in epilepsy (SUDEP). Incidence of SUDEP has been estimated to be 0.35 to 9.3/1000 person-years. Mechanisms of SUDEP are not clearly understood, it may include cardiac arrhythmia, myocardial ischemia, dysfunction of autonomic nervous system to the heart. Cardiac abnormalities are found in up to 33% at autopsy of SUDEP.14)
In our patient, apical RV hypokinesia was accompanied with LV involvement. Isolated LV involvement is the most common variant, but RV involvement is becoming well recognized. It has been reported that RV involvement affects approximately 25% to 42% of patients with TC.15) Daoko et al.5) said that most patients with biventricular TC are elderly women and presenting symptoms are similar to those of acute myocardial infarction. And it is known that RV involvement is associated with increasing hemodynamic instability and the risk of complications.
In conclusion, TC should form a part of the differential diagnoses of cardiac event in patient with seizure. In high risk patients, such as elevated plasma troponin level and hemodynamic instability is accompanied, ECG and TTE should be taken as soon as possible to detect TC and identify RV involvement. In a biventricular TC associated with epilepsy, more intensive and aggressive managements are required to prevent SUDEP and other complications.
Figures and Tables
Fig. 1
Chest radiograph showed cardiomegaly and patchy increased opacities in right upper and lower lobes.
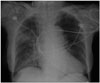
Fig. 3
Transthoracic echocardiography at admission. Apical ballooning of left ventricle was seen in diastolic apical 4 chamber view (A) and diastolic expanded apical 4 chamber (B). In diastolic modified apical 4 chamber view (C), right ventricle also seemed like ballooning of apex. In systole (D, E, and F) apex of right ventricle was hypokinetic (arrows) and basal right ventricle relatively seen to be over-contractile (arrows). LV: left ventricle, RV: right ventricular, RA: right atrium, LA: left atrium.
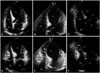
Fig. 4
Coronary angiography. There was no significant lesion of right coronary artery (RCA). In left coronary artery, ramus intermedius artery (RI) had significant stenosis, but left anterior descending artery (LAD) had no significant stenosis. LCX: left circumflex artery.
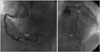
Fig. 5
Transthoracic echocardiography before discharge. In diastole (A and B), apex of left ventricle was not seen to be ballooning and contractility (arrow) of apical right ventricle was improved in systole (C and D). LV: left ventricle, RV: right ventricular, RA: right atrium, LA: left atrium, LVOT: left ventricular outflow tract.

References
1. Ako J, Sudhir K, Farouque HM, Honda Y, Fitzgerald PJ. Transient left ventricular dysfunction under severe stress: brain-heart relationship revisited. Am J Med. 2006; 119:10–17.
2. Ohtsuka T, Hamada M, Kodama K, Sasaki O, Suzuki M, Hara Y, Shigematsu Y, Hiwada K. Images in Cardiovascular Medicine. Neurogenic stunned myocardium. Circulation. 2000; 101:2122–2124.
3. Stöllberger C, Huber JO, Enzelsberger B, Finsterer J. Fatal outcome of epileptic seizure-induced takotsubo syndrome with left ventricular rupture. Eur J Neurol. 2009; 16:e116–e117.
4. Schneider F, Kadel C, Pagitz M, Sen S. Takotsubo cardiomyopathy and elevated troponin levels following cerebral seizure. Int J Cardiol. 2010; 145:586–587.
5. Daoko J, Rajachandran M, Savarese R, Orme J. Biventricular takotsubo cardiomyopathy: case study and review of literature. Tex Heart Inst J. 2013; 40:305–311.
6. Sato H, Tateishi H, Uchida T, Dote K, Ishihara M. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm. In : Kodama K, Haze K, Hori M, editors. Clinical aspect of myocardial injury: from ischemia to heart failure. Tokyo: Kagakuhyoronsya;1990. p. 56–64.
7. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006; 27:1523–1529.
8. Yun MH, Choi S, Park JY, Kim CH, Beom JW, Park G, Kim SH. Coincident takotsubo cardiomyopathy and coronary artery disease. Korean J Med. 2012; 83:791–795.
9. Yoshikawa T. Takotsubo cardiomyopathy, a new concept of cardiomyopathy: clinical features and pathophysiology. Int J Cardiol. 2015; 182:297–303.
10. Meierkord H, Shorvon S, Lightman SL. Plasma concentrations of prolactin, noradrenaline, vasopressin and oxytocin during and after a prolonged epileptic seizure. Acta Neurol Scand. 1994; 90:73–77.
11. Simon RP, Aminoff MJ, Benowitz NL. Changes in plasma catecholamines after tonic-clonic seizures. Neurology. 1984; 34:255–257.
12. Shimizu M, Kagawa A, Takano T, Masai H, Miwa Y. Neurogenic stunned myocardium associated with status epileptics and postictal catecholamine surge. Intern Med. 2008; 47:269–273.
13. Lee JW, Kim JY, Youn YJ, Sung JK, Lee NS, Lee KH, Yoo BS, Lee SH, Yoon J, Choe KH. Clinical characteristics and prognostic factors of stress-induced cardiomyopathy. Korean Circ J. 2010; 40:277–282.
14. Le Ven F, Pennec PY, Timsit S, Blanc JJ. Takotsubo syndrome associated with seizures: an underestimated cause of sudden death in epilepsy? Int J Cardiol. 2011; 146:475–479.
15. Haghi D, Athanasiadis A, Papavassiliu T, Suselbeck T, Fluechter S, Mahrholdt H, Borggrefe M, Sechtem U. Right ventricular involvement in Takotsubo cardiomyopathy. Eur Heart J. 2006; 27:2433–2439.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download