Abstract
The clinical diagnosis of right ventricular (RV) cardiomyopathies is often challenging. It is difficult to differentiate the isolated left ventricular (LV) noncompaction cardiomyopathy (NC) from biventricular NC or from coexisting arrhythmogenic ventricular cardiomyopathy (AC). There are currently few established morphologic criteria for the diagnosis other than RV dilation and presence of excessive regional trabeculation. The gross and microscopic changes suggest pathological similarities between, or coexistence of, RV-NC and AC. Therefore, the term arrhythmogenic right ventricular cardiomyopathy is somewhat misleading as isolated LV or biventricular involvement may be present and thus a broader term such as AC should be preferred. We describe an unusual case of AC associated with a NC in a 27-year-old man who had a history of permanent pacemaker 7 years ago due to second-degree atrioventricular block.
The clinical approach to right ventricular (RV) cardiomyopathies is often challenging.1)2) In 2006, we published the first clinical case report using echocardiography and magnetic resonance imaging to describe a syndrome of combined left ventricular (LV) noncompaction cardiomyopathy (NC) associated with arrhythmogenic ventricular cardiomyopathy (AC).3) However, it is difficult to differentiate the isolated LV-NC from biventricular NC or from coexisting AC. There are currently few established morphologic criteria for the diagnosis other than RV dilation and presence of excessive regional trabeculation.4) We describe an unusual case of AC associated with a NC in a 27-year-old man who had a history of permanent pacemaker 7 years ago due to second-degree atrioventricular block (AVB).
A 27-year-old man presented with repeated paroxysms of palpitations resulting from a rapid wide QRS complex tachycardia. He had a history of an AVB that had been treated by dual chamber permanent pacemaker at age of 20, and had not had any health problems since then. He reports having no palpitation at the time he received the permanent pacemaker. His family history was free of any cardiovascular pathology. The patient was in his normal state of health until approximately 6 months before admission, when he began suffering palpitation and presyncopal attacks. Clinical examination was unremarkable except for his pectus excavatum. On electrocardiogram (ECG), there were vertical spikes preceding each QRS complex, which had left bundle branch block (LBBB) morphology (Fig. 1A). This ECG is typical of RV pacing. However, ECG taken at emergency medicine showed incessant monomorphic ventricular tachycardia (VT) with a LBBB/inferior axis pattern (Fig. 1B). We planned an emergent catheter ablation to eliminate the frequent episodes of VT caused by electrical storms. During the endocardial electrophysiologic study under the guidance of 3-dimensional electroanatomic mapping using the Carto 3 system (The Carto 3 System, Biosense Webster, Waterloo, Belgium), the earliest ventricular activation signal during the arrhythmia, which preceded the onset of the QRS complex by 43 msec, was obtained from the RV outflow tract (Fig. 2). Activation mapping also demonstrated the arrhythmia focus and its propagation to the other sites of the RV. The classic entrainment with concealed fusion and best post-pacing interval was demonstrated and complete elimination of the VT was finally achieved with radiofrequency catheter ablation at that site. After the successful ablation of that site, a new and brief VT also detected but thought to be non-clinical VT; therefore it was not targeted.
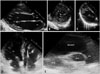
After the procedure and patients stabilization, we could performed transthoracic echocardiography; which was remarkable for the presence of diagnostic features of LV-NC, characteristically including a compacted epicardial layer and a noncompacted endocardial layer that consisted of a prominent trabecular meshwork and deep intertrabecular recesses filled with blood from the ventricular cavity in the apical and mid portions of the LV (Fig. 3A, B, and C, Supplementary movie 1). LV systolic function was preserved with ejection fraction of 65%. The RV was slightly dilated (RV, 31 mm in parasternal long axis view; RVOT, 41 mm in the parasternal long axis view) with exaggerated trabecular pattern (Fig. 3D and E) and mild systolic dysfunction. We could not perform a cardiac MRI (CMRI) due to his previous dual chamber rate-adaptive pacemaker and a endomyocardial biopsy because we estimated that the benefit/risk balance was unfavorable. The ECG taken after a pacemaker interrogation with lower heart rate (30 bpm/min) showed that 2:1 AVB (stars in Fig. 1C) with the epsilon (arrows in Fig. 1C and D) and negative T waves (Fig. 1). Therefore, it was decided to upgrade the dual-chamber pacemaker to dual-chamber implantable cardioverter defibrillator implantation. At present, this patient is being followed at the outpatient clinic.
Although there are rare cases of AC associated with LV-NC in literature,3)5)6)7) it has been debatable that it is a cardiomyopathy as a consequence of an underlying same genetic factor or a co-existence of two different entities.6)
Whereas the American Heart Association considers LV-NC to be a distinct primary genetic cardiomyopathy caused by the arrest of the normal compaction process of the developing myocardium,8) the European Society of Cardiology refers to LV-NC as an "unclassified cardiomyopathy" as it may merely be "a congenital or acquired morphological trait that is shared by many phenotypically distinct cardiomyopathies.9) Moreover, researches over the last decade have also shown poor agreement among the proposed diagnostic criteria for LV-NC.10) Zemrak et al.'s11) recent study and the accompanying editorial letter12) underline the need for demonstrably superior diagnostic criteria for LV-NC and suggest that a definitive LV-NC diagnosis should be established only in those patients who 1) fulfill a quantitative short-axis diagnostic criterion (Jenni criteria for echocardiography and Jacquier criteria for CMRI); and 2) also exhibit 1 of the following features: LV-NC diagnosed in another family member; regional wall motion abnormalities; LV-NC related complications (arrhythmia, heart failure, or thromboembolism); and being a carrier of a pathogenic mutation in a gene previously associated with LV-NC in various families.
On the other hand, the arrhythmogenic right ventricular cardiomyopathy (ARVC) raises many controversial issues with regard to its existence, defining and diagnostic criteria, pathologic spectrum of changes, and pathogenesis. Therefore, the term ARVC is somewhat misleading as biventricular involvement or isolated LV involvement may be present and thus broader term such as AC should be preferred.13) The recently modified actual diagnostic criteria for ARVC are often insufficient to make a diagnosis (low sensitivity, high specificity).1) Indeed, it is difficult to differentiate the isolated LV-NC from biventricular NC or from coexisting AC.2) There are currently few established morphologic criteria for the NC diagnosis other than RV dilation and presence of excessive regional trabeculation. The gross and microscopic changes suggest pathological similarities between, or coexistence of RV-NC and AC.1)4)
Another important finding in this current report was that the reason of the previous syncope was related to AVB rather than VT. In patients with AC and syncope beyond detectable or inducible monomorphic VT, several mechanisms of syncope could be identified with conduction disease as the predominant finding.14) The autopsy findings have shown that specific RV wall abnormalities are associated primarily with His bundle15)16) and LV abnormalities.16) In an autopsy study of 200 consecutive AC cases, the His bundle and its branches were abnormal in most cases (68%), either because of infiltration of adipose tissue (8%), fibrosis (54%), or both (6%).16) On the other hand; whereas histologically conductive tissue infiltration in AC was prevalent (≈70%), clinically AVB is about 6%,16) and appears as the tip of the iceberg of the conduction tissue involvement.
In conclusion, it is yet to be resolved as to whether isolated NC occurs as a distinctive pathological diagnosis or only in association with AC or with obligatory biventricular involvement.4) Further investigations will be necessary to establish diagnostic criteria and determine whether isolated biventricular NC is same or different entity from AC.
Figures and Tables
Fig. 1
The 12-lead electrocardiogram showing (A) the typical of right ventricular pacing pattern, (B) the monomorphic ventricular tachycardia with a LBBB/inferior axis pattern; (C) 2:1 atrioventricular block (stars in C) with the epsilon (arrows in C and D) and negative T waves. LBBB: left bundle branch block.
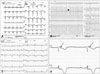
Fig. 2
The endocardial bipolar electroanatomical mapping showing the earliest ventricular breaktrought site (A) and the scar area at preferentially RVOT (A) and a less extent degree at LVOT (B and C). RV: right ventricle, LV: left ventricle, RVOT: right ventricular outflow tract, LVOT: left ventricular outflow tract.
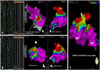
Fig. 3
The transthoracic echocardiography showing a compacted epicardial layer and a noncompacted endocardial layer that consisted of a prominent trabecular meshwork and deep intertrabecular recesses filled with blood from the ventricular cavity in the apical and mid portions of the LV (A-D), and an enlargement of RVOT with exaggerated trabecular pattern within the RV (D and E). RV: right ventricle, LV: left ventricle, LA: left atrium, RA: right atrium, RVOT: right ventricular outflow tract, PA: pulmonary artery, Ao: aorta.
References
1. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010; 121:1533–1541.
2. Wlodarska EK, Wozniak O, Konka M, Piotrowska-Kownacka D, Walczak E, Hoffman P. Isolated ventricular noncompaction mimicking arrhythmogenic right ventricular cardiomyopathy--a study of nine patients. Int J Cardiol. 2010; 145:107–111.
3. Tufekcioglu O, Aras D, Sahin O, Ergun K, Hazirolan T, Celenk MK, Demir AD. Two cardiomyopathies in one heart. Echocardiography. 2006; 23:519–521.
4. Ilyas S, Ganote C, Lajoie D, Robertson J, Cline-Parhamovich K. Sudden death and isolated right ventricular noncompaction cardiomyopathy: report of 2 autopsied adult cases. Am J Forensic Med Pathol. 2013; 34:225–227.
5. Matsukuma S, Eishi K, Hashizume K, Oshitomi T, Ariyoshi T, Taniguchi S, Hisatomi K, Hayashi T, Abe K. Arrhythmogenic left ventricular cardiomyopathy associated with noncompaction. Ann Thorac Surg. 2010; 90:2044–2046.
6. Song ZZ. A combination of right ventricular hypertrabeculation/noncompaction and arrhythmogenic right ventricular cardiomyopathy: a syndrome? Cardiovasc Ultrasound. 2008; 6:63.
7. Honarbakhsh S, Suman-Horduna I, Mantziari L, Ernst S. Arrhythmogenic Right Ventricle in Left Ventricular Non-compaction - In response to "Right Ventricular Ablation as a Therapeutic Option for Left Ventricular Hypertrabeculation / noncompaction". Indian Pacing Electrophysiol J. 2014; 14:105–107.
8. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. American Heart Association. Council on Clinical Cardiology, Heart Failure and Transplantation Committee. Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups. Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006; 113:1807–1816.
9. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008; 29:270–276.
10. Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, McKenna WJ, Sharma S, Elliott PM. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J. 2008; 29:89–95.
11. Zemrak F, Ahlman MA, Captur G, Mohiddin SA, Kawel-Boehm N, Prince MR, Moon JC, Hundley WG, Lima JA, Bluemke DA, Petersen SE. The relationship of left ventricular trabeculation to ventricular function and structure over a 9.5-year follow-up: the MESA study. J Am Coll Cardiol. 2014; 64:1971–1980.
12. Garcia-Pavia P, de la. Left ventricular noncompaction: a genetic cardiomyopathy looking for diagnostic criteria. J Am Coll Cardiol. 2014; 64:1981–1983.
13. Saguner AM, Brunckhorst C, Duru F. Arrhythmogenic ventricular cardiomyopathy: A paradigm shift from right to biventricular disease. World J Cardiol. 2014; 6:154–174.
14. Peters S, Trümmel M, Koehler B, Westermann KU. Mechanisms of syncopes in arrhythmogenic right ventricular dysplasia-cardiomyopathy beyond monomorphic ventricular tachycardia. Int J Cardiol. 2006; 106:52–54.
15. Beerman LB, Zuberbuhler JR, Neches WH, Fischer DR, Fricker FJ, Mathews RA, Park SC, Lenox CC. Arrhythmogenic right ventricular dysplasia associated with atrioventricular conduction disturbance. Am J Cardiol. 1983; 52:909–912.
16. Tabib A, Loire R, Chalabreysse L, Meyronnet D, Miras A, Malicier D, Thivolet F, Chevalier P, Bouvagnet P. Circumstances of death and gross and microscopic observations in a series of 200 cases of sudden death associated with arrhythmogenic right ventricular cardiomyopathy and/or dysplasia. Circulation. 2003; 108:3000–3005.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download