Abstract
Background
Methods
Results
Figures and Tables
Fig. 2
Histogram of the distribution of baseline global longitudinal strain rate on apical 4 chamber image (-14.70 ± 4.46%, range -23.9 - -6.3%, frame rate 34.63 ± 14.31/sec, heart rate).

Fig. 3
Receiver operating characteristic curve showing the best cutoffs for prediction of all-cause mortality in patients with chronic aortic regurgitation. GS-4CH: global strain rate on apical four chamber image, LVEF: left ventricular ejection fraction, LVEDD: left ventricular end-diastolic diameter, AUC: area under the curve, CI: confidence interval.

Fig. 4
Kaplan-Meier curve for all-cause mortality stratified by GS-4CH in all patients with chronic AR (A) and patients received AVR (B). GS-4CH: global strain rate on apical four chamber image, AR: aortic regurgitation, AVR: aortic valve replacement.

Table 1
Baseline characteristics of patients with chronic aortic regurgitation
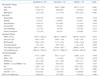
BSA: body surface area, IHD: ischemic heart disease, WBC: white blood cell, BUN: blood urea nitrogen, NT-proBNP: N-terminal-pro B-type natriuretic peptide, LVEF: left ventricular ejection fraction, LVEDD: left ventricular end-diastolic diameter, LVESD: left ventricular end-systolic diameter, GS-4CH: global strain rate on apical four chamber image
Table 2
Baseline characteristics according to treatment
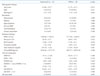
AVR: aortic valve replacement, BSA: body surface area, IHD: ischemic heart disease, WBC: white blood cell, BUN: blood urea nitrogen, NT-proBNP: N-terminal-pro B-type natriuretic peptide, LVEF: left ventricular ejection fraction, LVEDD: left ventricular end-diastolic diameter, LVESD: left ventricular end-systolic diameter, GS-4CH: global strain rate on apical four chamber image
Table 3
Baseline characteristics according to global strain

GS-4CH: global strain rate on apical four chamber image, BSA: body surface area, IHD: ischemic heart disease, WBC: white blood cell, BUN: blood urea nitrogen, NT-proBNP: N-terminal-pro B-type natriuretic peptide, LVEF: left ventricular ejection fraction, LVEDD: left ventricular end-diastolic diameter, LVESD: left ventricular end-systolic diameter
Table 4
Multivariate analysis on Cox proportional hazard model for all-cause mortality in patients with chronic aortic regurgitation

CI: confidence interval, BSA: body surface area, AF: atrial fibrillation, BUN: blood urea nitrogen, LVEF: left ventricular ejection fraction, LVEDD: left ventricular end-diastolic diameter, LVESD: left ventricular end-systolic diameter, AVR: aortic valve replacement, GS-4CH: global strain rate on apical four chamber image




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download