Abstract
Although stress-induced cardiomyopathy (SCMP) is a reversible disease and the prognosis is usually excellent, several complications can occur and can result in fatal adverse events. The formation of left ventricular (LV) thrombus is one of these critical complications of SCMP. This report describes a case of SCMP complicated by formation of a LV thrombus that became increasingly mobile as LV contractility recovered, and for which surgical removal was performed. Here, we report a case of SCMP complicated by LV thrombus and review the literature regarding this topic.
Stress-induced cardiomyopathy (SCMP) is a nonischemic reversible cardiomyopathy characterized uniquely by a left ventricular (LV) wall motion abnormality termed "apical ballooning" and the prognosis is generally excellent.1)2) However, several critical complications are possible. The formation of LV thrombus is one of these rare complications which can result in systemic embolism.3) Here, we report a case of SCMP complicated by LV mural thrombus which gradually increased in mobility as LV contractility recovered and which eventually required surgical removal.
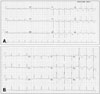
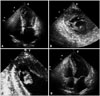
A 66-year-old woman who presented with severe dysphagia visited our emergency room. On admission her mental status was alert, and she had a blood pressure of 115/76 mm Hg, a respiratory rate of 20 per minute, a pulse rate of 96 per minute, and a body temperature of 36.4℃. She did not have any history of heart disease, hypertension or diabetes mellitus, but had undergone subtotal gastrectomy due to stomach cancer. Urgent gastrofibroscopy was performed and a piece of chicken trapped at the lower esophagus was removed. Additionally, a small polyp was found at the previous surgical anastomosis site which subsequently confirmed pathologically as early gastric cancer. The patient also complained of mild dyspnea and diffuse chest discomfort on admission, and an electrocardiogram (ECG) showed ST elevation with QT prolongation (QTc = 480 msec), and T wave inversion on leads V2-6 (Fig. 1A). Troponin T and creatinine kinase-MB (CK-MB) levels were elevated to 0.29 ng/mL and 20.46 ng/mL, respectively (reference range; < 0.01 ng/dL for troponin T, < 3.61 ng/mL for CK-MB). Transthoracic echocardiography (TTE) revealed mid and apical LV segmental wall motion abnormalities with apical ballooning that did not correspond to a coronary artery territory, as well as a left ventricular ejection fraction of 41% (LV end-diastolic volume/LV end-systolic volume = 84/49 mL) (Fig. 2A). In addition, a 19 × 18-mm-sized non-mobile echogenic mass suspicious for a mural thrombus was found at the apex of the left ventricle (Fig. 2B). As the features were consistent with SCMP complicated by LV mural thrombus, anticoagulation as well as conventional heart failure therapy was initiated. After one week, ST elevation disappeared on follow-up ECG (Fig. 1B) and follow-up TTE demonstrated resolution of the mid and apical LV segmental wall motion abnormalities and fully recovered LV systolic function. However, the LV mural thrombus had partially detached from the LV wall with recovery of LV contractility and was adherent to the ventricular wall by a narrow stalk. The remarkably increased mobility of the LV thrombus was thought to carry a very high thromboembolic risk (Fig. 2C). Thus, surgical removal of the thrombus was decided upon to prevent embolic complications.
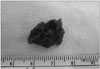
Oblique aortotomy was performed under support of cardiopulmonary bypass. The mass, which had its base attached to the trabecular endocardium of the apico-inferior wall of the LV cavity, was completely removed by suction tip through the aortic valve. The mass appeared to be an amorphous bizarre shape, measuring 1.7 × 1.2 × 0.7 cm in size (Fig. 3). On subsequent pathohistological examination, the mass was confirmed to be a thrombus.
On postoperative day 7, follow-up TTE was performed. LV systolic function was normal without any regional wall motion abnormalities (Fig. 2D). There was no evidence of residual thrombus or signs of systemic embolism. The patient recovered without any other complications and was discharged 18 days after the operation.
SCMP was first described in Japan in 1991.1) It is characterized by a reversible LV systolic dysfunction with distinct 'apical ballooning' accompanied by slightly increased cardiac enzyme levels and electrocardiographic changes including QT prolongation, ST elevation, or T wave inversion. Despite many investigations, the mechanism of SCMP is not yet fully understood and many investigators have suggested a catecholamine-mediated mechanism as the cause of SCMP.2) Although the long term prognosis of patients with SCMP is usually excellent,3) well-known complications of SCMP are cardiogenic shock, congestive heart failure and sudden cardiac death. Besides these morbidity, ventricular tachycardia, LV rupture, apical thrombus formation and embolism have been reported as rare complications of SCMP.3) According to previous studies, the prevalence of LV thrombus in cases of SCMP is approximately 2.5%, and the occurrence rate of embolic complications in this condition is reported to be as high as 33% despite proper anticoagulation therapy.4)5) The pathogenesis of LV thrombus formation in SCMP is still not fully known and some theories have been suggested hemostasis,6) catecholamine induced platelet activation,7)8) and catecholamine induced cardiomyocyte damage9) as underlying mechanism.
Anterior acute myocardial infarction (AMI) can also be complicated by LV thrombus. The incidence of LV thrombus formation in anterior AMI was reported to be 30-40% before the era of reperfusion therapy,10)11) and the embolic rate of the condition was 10-15%.12) The relatively low incidence of LV thrombus formation (2.5% vs. 30-40%) and high embolic risk (33% vs. 10-15%) in SCMP compared to AMI might be related to faster and better recovery of LV function. As LV wall motion recovers, the mobility of mural thrombus may increase rapidly by partial detachment from the ventricular wall, which can lead to increased embolic risk. In SCMP, the mural thrombus that was initially immobile can become highly mobile within a short time. And this phenomenon can explain why initially mobile LV thrombus in SCMP is not associated with a higher embolic event rate than initially immobile LV thrombus in previous studies.5) Of 11 previously reported cases of SCMP that were complicated by a mobile LV thrombus, LV thrombus was immobile or even absent in the initial examination of 6 cases.13)14)15)16)17)18)19)20) Thus, the embolic risk of LV thrombus in SCMP may not be determined based on the initial mobility of the thrombus.
In all reported cases with SCMP, anticoagulation therapy was promptly initiated after the detection of the thrombus. Despite appropriate anticoagulation therapy, however, cardiac embolic events occurred in one third of patients with SCMP complicated by LV thrombus. In contrast, when the thrombus was removed by surgery using a trans-apical approach there was no embolic event in two reported cases.19)20) In the present case, a trans-aortic approach following oblique aortotomy was performed. Although the apical approach can provide good and easy access to the thrombus, myocardial injury is inevitable. Using a trans-aortic approach following aortotomy, visualization or extraction of the thrombus through the aortic valve may be somewhat difficult, but myocardial damage can be avoided, and this approach may be more beneficial for the heart, especially in a clinical setting.
To the best of our knowledge, this is the first reported case of SCMP complicated by LV thrombus which was surgically removed by a trans-aortic approach after oblique aortotomy. Although LV thrombus is a rare complication of SCMP, the embolic risk is relatively high because of rapid change of thrombus mobility. Therefore, short-term TTE follow-up after anticoagulation therapy might be recommended in this clinical setting, and if the thrombus becomes highly mobile as LV systolic function improves, surgical removal could be a reasonable therapeutic option.
Figures and Tables
Fig. 1
Electrocardiogram on admission showing ST elevation and T wave inversion in the precordial leads and a prolonged QT interval (480 msec) (A). ST elevation disappeared after one week on follow-up electrocardiogram (B).
References
1. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases]. J Cardiol. 1991; 21:203–214.
2. Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008; 5:22–29.

3. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004; 141:858–865.

4. de Gregorio C, Grimaldi P, Lentini C. Left ventricular thrombus formation and cardioembolic complications in patients with Takotsubo-like syndrome: a systematic review. Int J Cardiol. 2008; 131:18–24.

5. de Gregorio C. Cardioembolic outcomes in stress-related cardiomyopathy complicated by ventricular thrombus: a systematic review of 26 clinical studies. Int J Cardiol. 2010; 141:11–17.

6. van Dantzig JM, Delemarre BJ, Bot H, Visser CA. Left ventricular thrombus in acute myocardial infarction. Eur Heart J. 1996; 17:1640–1645.

7. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005; 352:539–548.

8. Ardlie NG, Glew G, Schwartz CJ. Influence of catecholamines on nucleotide-induced platelet aggregation. Nature. 1966; 212:415–417.

9. Mann DL, Kent RL, Parsons B, Cooper G 4th. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992; 85:790–804.

10. Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol. 1989; 14:903–911.

11. Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med. 1984; 100:789–794.

12. Stratton JR, Resnick AD. Increased embolic risk in patients with left ventricular thrombi. Circulation. 1987; 75:1004–1011.

13. Singh V, Mayer T, Salanitri J, Salinger MH. Cardiac MRI documented left ventricular thrombus complicating acute Takotsubo syndrome: an uncommon dilemma. Int J Cardiovasc Imaging. 2007; 23:591–593.

14. de Gregorio C, Cento D, Di Bella G, Coglitore S. Minor stroke in a Takotsubo-like syndrome: a rare clinical presentation due to transient left ventricular thrombus. Int J Cardiol. 2008; 130:e78–e80.

15. Haghi D, Papavassiliu T, Heggemann F, Kaden JJ, Borggrefe M, Suselbeck T. Incidence and clinical significance of left ventricular thrombus in tako-tsubo cardiomyopathy assessed with echocardiography. QJM. 2008; 101:381–386.

16. Wakabayashi K, Dohi T, Daida H. Takotsubo cardiomyopathy associated with epilepsy complicated with giant thrombus. Int J Cardiol. 2011; 148:e28–e30.

17. Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nakama Y, Maruhashi T, Kagawa E, Dai K. Incidence and treatment of left ventricular apical thrombosis in Tako-tsubo cardiomyopathy. Int J Cardiol. 2011; 146:e58–e60.

18. Matsuzono K, Ikeda Y, Deguchi S, Yamashita T, Kurata T, Deguchi K, Abe K. Cerebral embolic stroke after disappearing takotsubo cardiomyopathy. J Stroke Cerebrovasc Dis. 2013; 22:e682–e683.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download