Abstract
Although pulmonary artery angiosarcoma is rare, it can be misdiagnosed as pulmonary embolism because of its similar clinical and diagnostic features. The diagnosis is often delayed and the misdiagnosis brings unnecessary treatment. Because we made a wrong diagnosis of pulmonary artery angiosarcoma as an acute pulmonary embolism, we did thrombolytic therapy which could be dangerous to the patient. In this case report, we focused on the clinical and echocardiographic features of pulmonary artery angiosarcoma which can be used in differentiating the diagnosis from pulmonary embolism.
Because echocardiographic examination gives us anatomic and functional information of the heart and vessels, it is the major screening tool in various cardiovascular diseases. However, it is difficult to differentiate the etiologies if patients present with similar clinical and echocardiographic characteristics. We experienced a case with pulmonary artery angiosarcoma, initially confused with pulmonary thromboembolism (PTE). Because we did not diagnose the pulmonary artery angiosarcoma, we did thrombolytic therapy which could be dangerous to the patient. In this case report, we focused on the clinical and echocardiographic features of pulmonary artery angiosarcoma which can be used in differentiating the diagnosis from PTE.
A 65-year-old man was admitted to our hospital complaining of exertional dyspnea for 10 days. He had been medicated with oral antihypertensive drugs for 4 years. He discontinued his medications by himself due to the dropping of systolic blood pressure to 90 mm Hg 7 days ago. On the admission, his blood pressure was 130/90 mm Hg and his pulse was regular at 66 beats per minute. The transthoracic echocardiogram (TTE) showed a dilated right ventricle (RV) with marked RV systolic dysfunction (Fig. 1A), D-shaped left ventricle (Fig. 1B), and showed a large mass, about 50 × 30 mm sized, considered to be a thrombus between the right ventricular outflow tract (RVOT) and the main pulmonary artery (MPA) (Fig. 1C). The systolic RV pressure measured by maximal tricuspid regurgitation velocity (TR Vmax of 5.2 m/sec) was about 110 mm Hg. TTE also revealed mass lesion mimicking thrombus in the MPA with severe stenotic component (measured maximal MPA velocity of 4.6 m/sec) (Fig. 1D). Spiral computed tomography (CT) of the chest demonstrated an intraluminal filling defect, about 55 × 31 mm sized, occupying the lumen of the MPA (Fig. 2A) and multiple filling defects of the peripheral pulmonary arteries. Although the patient had normal blood pressure, he was initially treated with intravenous recombinant tissue plasminogen activator (rt-PA, 100 mg for 2 hours) to improve his severely decreased RV systolic function.
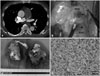
Despite receiving two days of treatment with thrombolytic and anticoagulation therapy, the patients showed little improvement. The follow-up TTE still demonstrated similar features of the previous echocardiographic examination. Dyspnea of the patient aggravated from New York Heart Association functional class II to III. After a thoughtful review of the follow-up TTE, we found several differences that differentiated from the usual echocardiograms of PTE. The mass filled the proximal portion of the MPA with involvement of the pulmonary valves. The mass was also attached to the anterior portion of the RVOT. CT revealed intraluminal filling defect occupying the entire lumen of the MPA and an extraluminal extension to the anterior wall of the RVOT. The attending physician suspected the mass of the MPA to be a true mass, not a thrombus, and decided to perform an open heart surgery to remove the mass. Three days after the thrombolysis, the patient underwent the operation. The surgeon noted that the tumor extended from the RVOT to the proximal part of both pulmonary arteries (Fig. 2B). Additionally, the pulmonary valves were invaded by tumor tissue (Fig. 2C). After radical removal of the mass, the patient had pulmonary valve replacement therapy with prosthetic valve.
The pathological examination revealed malignant infiltration with about 50% of necrotic areas, markedly increased cellularity and pleomorphic spindle cells. The mitotic activity was high (20/10 high power field) (Fig. 2D). Immunohistochemical studies demonstrated that CD31 (an endothelial marker) and vimentin (a mesenchymal marker) were positive while desmin was negative. Based on these findings, the pathologist diagnosed pulmonary artery angiosarcoma. The patient was treated with 4 sessions of antitumor chemotherapy with combination of mesna, doxorubicin, ifosfamide, and dacarbazine after the operation. He has no evidence of local recurrence or distant metastasis 12 months after the operation.
Pulmonary artery angiosarcoma is a rare malignancy and its incidence is about 3.6% of pulmonary artery sarcomas.1) The prognosis of pulmonary artery angiosarcoma is extremely poor if a complete resection of the tumor is not feasible. The median survival without resection is about 1.5 months.2) The usual cause of death is heart failure, predominantly right-sided, as a result of RVOT obstruction. Metastasis are typically mainly to the lung and other organs including lymph nodes, liver, pancreas, kidneys and mesentery.3) Recently, there was a report that showed an improved survival probably due to aggressive resection with curative intent and multimodality therapy.4) Moreover, the detection of tumor can reduce the unnecessary use of thrombolytic therapy which might be dangerous to patients. So, the precise diagnosis is vital to improve the survival in patients with pulmonary artery angiosarcoma.
Although pulmonary artery angiosarcoma is rare, it can be misdiagnosed as PTE because of its similar clinical and radiological features.1)5)6) The diagnosis is often delayed and is difficult to avoid misdiagnosis. Because pulmonary artery angiosarcoma grows gradually, it usually has a long asymptomatic course and insidious onset of symptoms of pulmonary arterial obstruction.7) Pulmonary artery angiosarcoma is frequently confused with a chronic form of PTE.
However, the presentation of our case was like an acute PTE, so we had a problem in the diagnosis. We found several different clinical findings of our case compared to the usual forms of acute PTE.
First of all, our case had a low risk of developing deep vein thromboembolism and there was no sign of unilateral swelling of the leg. Because deep vein thrombosis is the major contributing factor of developing PTE, the clinical probability of PTE was low in this patient.
Second, the patient had different echocardiographic patterns. His echocardiographic examination showed a bulging mass lesion impacted in the MPA which was different from linear mass lesions usually shown in acute PTE. When thrombi are visualized in the MPA in patients with acute PTE, they are usually located in its distal part near the bifurcation. They extend to right and/or left pulmonary artery in many cases.8)9) The involvement of the pulmonary valves and the attachment to the RVOT were other differential findings suggesting a malignant mass with infiltration. Because TTE has no harmful effect, the short-term follow-up echocardiographic examination can be an attractive method for the differential diagnosis. In our case, there was no change after thrombolytic and anticoagulant therapy in the follow-up echocardiogram. It gave us useful information in the diagnosis.
Third, the chest CT demonstrated an intraluminal filling defect occupying the entire lumen of the MPA and an extraluminal extension to the anterior wall of the RV. This finding was compatible with the surgical finding. According to several studies on the role of CT in differentiating these two disease categories, the characteristics of CT findings that favor the diagnosis of pulmonary artery sarcomas rather than of PTE include a low-attenuation filling defect, heterogeneous enhancement of mass occupying the entire luminal diameter of the main or the proximal pulmonary artery, and extravascular spread of the lesion.10)
In conclusion, physicians should consider the possibility of pulmonary artery angiosarcoma as a differential diagnosis of acute PTE, especially in patients with low or intermediate probability of PTE. The echocardiographic features, including proximal location of mass in MPA, involvement of pulmonary valves, and no significant change in the short-term follow-up echocardiography, can give additional information in the differential diagnosis.
Figures and Tables
Fig. 1
Modified apical 4 chamber view shows dilated RV (A) and parasternal short axis view demonstrates D-shaped left ventricle indicating pressure overload of RV (B). Parasternal short axis view of the main pulmonary artery (MPA) reveals a mass lesion, about 50 × 30 mm sized, in the proximal part of the MPA with involvement of the pulmonary valves (arrow, C). The color Doppler imaging of the MPA shows flow acceleration during systole and the measured maximal velocity was about 4.6 m/sec (D). Ao: aorta, LV: left ventricle, RV: right ventricle.
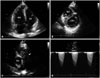
Fig. 2
Chest computerized tomographic scan demonstrates a large, about 58 × 31 mm sized, filling defect occupying the entire MPA with involvement of the pulmonary valves (arrow, A). Surgical finding shows the involvement of the anterior part of MPA and the RVOT (arrowheads, B). Resected tumor masses from the MPA and the RVOT demonstrates several hemorrhagic foci (C). It was divided into two separate parts during removal of the mass. The pathologic specimen demonstrated multiple pleomorphic spindle cells with mitotic figures indicating malignancy (hematoxylin and eosin stain, × 400, D). MPA: main pulmonary artery, RVOT: right ventricular outflow tract.
References
1. Nonomura A, Kurumaya H, Kono N, Nakanuma Y, Ohta G, Terahata S, Matsubara F, Matsuda T, Asaka T, Nishino T. Primary pulmonary artery sarcoma. Report of two autopsy cases studied by immunohistochemistry and electron microscopy, and review of 110 cases reported in the literature. Acta Pathol Jpn. 1988; 38:883–896.
2. Anderson MB, Kriett JM, Kapelanski DP, Tarazi R, Jamieson SW. Primary pulmonary artery sarcoma: a report of six cases. Ann Thorac Surg. 1995; 59:1487–1490.
3. Bleisch VR, Kraus FT. Polypoid sarcoma of the pulmonary trunk: analysis of the literature and report of a case with leptomeric organelles and ultrastructural features of rhabdomyosarcoma. Cancer. 1980; 46:314–324.
4. Blackmon SH, Rice DC, Correa AM, Mehran R, Putnam JB, Smythe WR, Walkes JC, Walsh GL, Moran C, Singh H, Vaporciyan AA, Reardon M. Management of primary pulmonary artery sarcomas. Ann Thorac Surg. 2009; 87:977–984.
5. Tschirch FT, Del Grande F, Marincek B, Huisman TA. Angiosarcoma of the pulmonary trunk mimicking pulmonary thromboembolic disease. A case report. Acta Radiol. 2003; 44:504–507.
6. Huo L, Lai S, Gladish G, Czerniak BA, Moran CA. Pulmonary artery angiosarcoma: a clinicopathologic and radiological correlation. Ann Diagn Pathol. 2005; 9:209–214.
7. Cox JE, Chiles C, Aquino SL, Savage P, Oaks T. Pulmonary artery sarcomas: a review of clinical and radiologic features. J Comput Assist Tomogr. 1997; 21:750–755.
8. Wittlich N, Erbel R, Eichler A, Schuster S, Jakob H, Iversen S, Oelert H, Meyer J. Detection of central pulmonary artery thromboemboli by transesophageal echocardiography in patients with severe pulmonary embolism. J Am Soc Echocardiogr. 1992; 5:515–524.
9. Oser RF, Zuckerman DA, Gutierrez FR, Brink JA. Anatomic distribution of pulmonary emboli at pulmonary angiography: implications for cross-sectional imaging. Radiology. 1996; 199:31–35.
10. Yi CA, Lee KS, Choe YH, Han D, Kwon OJ, Kim S. Computed tomography in pulmonary artery sarcoma: distinguishing features from pulmonary embolic disease. J Comput Assist Tomogr. 2004; 28:34–39.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download