Abstract
A 31-year-old male who had been treated for Churg-Strauss syndrome (CSS) presented with sudden onset of dysarthria. Brain magnetic resonance imaging (MRI) showed acute multifocal bilateral cerebral infarctions suggesting embolic causes. Cardiac MRI showed dilated cardiomyopathy with severe biventricular dysfunction with intracardiac thrombi, and multiple high signal intensity spots in myocardium of the left ventricle with multifocal delayed enhancement suggesting multifocal myocarditis due to small vessel vasculitis associated with CSS. After anticoagulation therapy, treatments for heart failure, and immunosuppressive therapy including parenteral steroids and cyclophosphamide to control CSS, the symptoms and signs of heart failure and cardiac function of the patient were improved. Considering the pathophysiologic mechanism of cardiac involvement in CSS, immunosuppressive therapy to control the disease activity of CSS should be taken into account, besides usual management for heart failure.
Churg-Strauss syndrome (CSS) is a rare multi-systemic vasculitis disorder, and cardiac involvement is known to be associated with very poor prognosis, accounting for approximately one-half of deaths.1) However, there has been limited information on the proper diagnosis and management of cardiac involvement of CSS.
Here, we report a cardiac involvement case of CSS presenting as dilated cardiomyopathy (DCMP) and complicated by multiple cerebral infarctions. DCMP of the present case was reversible after 3 months of appropriate medical managements including immunosuppressive therapy.
A 31-year-old male consulted from the neurology department to evaluate the cardioembolic source of multifocal cerebral infarctions (Fig. 1) and progressive dyspnea. He had been treated for CSS with eosinophilic pneumonia, bronchial asthma, and paranasal sinusitis for last ten years from the department of allergic disease. His blood pressure was 120/70 mm Hg, heart rate was 96/min, body temperature was 96.7℃. And, his electrocardiogram (ECG) on admission showed normal sinus rhythm (Fig. 2A). Crackles on both lung fields, jugular venous distention, and pitting edema of both legs were noted on physical examination.
Blood chemistries showed marked elevation of liver enzymes with mild hyperbilirubinemia, hypereosinophilia (1500/mm3), the increased level of C-reactive protein (19.5 mg/dL), and normal ranged troponin I. N-terminal-pro-B-type natriuretic peptide was also markedly increased (5135 pg/mL).
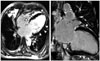
Chest X-ray showed cardiomegaly and bilateral pulmonary opacities (Fig. 3A). Echocardiography revealed dilated all cardiac chambers, global hypokinesia with severe biventricular dysfunction (ejection fraction: 20%) (Fig. 3B), moderate degree of functional mitral and tricuspid regurgitation with severe pulmonary hypertension (estimated pulmonary artery systolic pressure = 78.8 mm Hg). Restrictive filling pattern of mitral inflow was noted on diastolic functional evaluation (Fig. 3C). Two mobile and hyperechoic thrombi were also observed within the left ventricular (LV) apex (Fig. 3D). To evaluate the underlying cause of DCMP and cardiac involvement of CSS, cardiac magnetic resonance imaging (MRI) was performed. Cardiac MRI revealed multiple high signal intensity spots in the LV myocardium with multifocal delayed enhancement, mainly subendocardial area, suggesting the multifocal myocarditis due to the small vessel vasculitis associated with CSS. Consolidative lesions with high signal intensity in right middle lobe, suggesting pulmonary involvement of CSS, were also noted (Fig. 4).
With the diagnosis of acute decompensated heart failure due to DCMP complicated by LV thrombosis and subsequent acute embolic cerebral infarctions in CSS, anticoagulation therapy, treatments for heart failure, and immunosuppressive therapy including oral steroids (Methylon, Kunwha Pharm, Seoul, Korea, methylprednisolon 24 mg for one month) and parenteral cyclophosphamide (Endoxan, Bukwang Pharm, Seoul, Korea, cyclophosphamide 1000 mg for 1 day) to control the disease activity of CSS were done. The symptoms and signs of heart failure, and neurologic deficits were gradually disappeared. His electrocardiogram at discharge was shown in Fig. 2B. After 3 months of medical therapy, cardiomegaly and pulmonary infiltrates were disappeared on follow-up chest X-ray (Fig. 3E). Follow-up echocardiography showed improved wall motion and function of both ventricles with the normalization of the cardiac chamber size, and the LV thrombi were also disappeared (Fig. 3F, G, and H).
Here we report a case of multiple cerebral infarction in a young male who met 5 diagnostic criteria of CSS established by American College of Rheumatology,2) including a history of severe asthma, recurrent sinusitis, eosinophilia, pulmonary infiltrates, lung biopsy containing a blood vessel with extravascular eosinophils, and had no classical cardiovascular risk factors. The present case has several clinically important messages in the treatment of CSS. Firstly, DCMP may develop as an unusual form of cardiac involvement in the course of CSS, and the DCMP associated with CSS may be reversible by appropriate medical managements. Secondly, the use of multi-modality imaging including echocardiography and cardiac MRI would be essential in the diagnosis of cardiac involvement of CSS, and it may replace the role of cardiac biopsy which are invasive. Thirdly, considering the pathophysiologic mechanism of DCMP in CSS, the use of immunosuppressive therapy to control the activity of CSS might be warranted, even in patients with depressed LV systolic function.
Cardiac involvement of CSS has been reported as a form of non specific conduction disturbance, peri-myocarditis, pericardial effusion, cardiac tamponade, or myocardial infarction due to vasculitis of coronary vessels.3) And pulmonary hypertension secondary to concomitant pulmonary involvement may also induce right ventricular dysfunction. However, heart failure associated with DCMP is uncommon, and only few cases have been described.4)5) In this situation, intracardiac thrombi complicated by multiple embolic cerebral infarction can be developed. Although neurologic involvement is common in CSS, the involvement of central nervous system is uncommon.6) Stroke has been reported only in less than 10% of patients with CSS,7)8) and the cause of cerebral infarction were mostly attributable to the vasculitic complications of cerebral arteries. In the present case, however, the cause of cerebral infarction was the embolism of intracardiac thrombi which developed as a complication of DCMP. Therefore, intracardiac source of embolism should be searched in CSS patients with neurologic symptoms.
The diagnosis of cardiac involvement in patients with CSS is not easy because of lack of symptoms. Although the present case presented with typical symptoms of heart failure, cardiac involvement may be detected in about 40% of the patients with CSS who have no symptoms or ECG abnormality,9) indicating that the absence of symptoms or abnormal ECG cannot exclude cardiac involvement. Therefore, it is recommended that the evaluation for cardiac involvement in patients with CSS should include not only detailed history of cardiac symptoms and ECG, but also multi-modality imaging with echocardiography or cardiac MRI. Recent studies have demonstrated that cardiac MRI might be a useful imaging modality for early detection and serial follow-up of cardiac involvement of CSS.10)11) Cardiac MRI may replace the role of cardiac biopsy or even be a better tool than cardiac biopsy in evaluating cardiac involvement of CSS because cardiac biopsy is an invasive method and can demonstrate myocardial involvement of the limited portion of the heart. Cardiac MRI, whereas, can demonstrate not only the involvement of whole myocardium of all cardiac chambers, but also pericardial involvement. Typical cardiac MRI findings, as shown in the present case, include high signal intensity of myocardium with multifocal delayed enhancement, mainly subendocardial area, suggesting the multifocal myocarditis due to the small vessel vasculitis.12)
As in other known reversible causes of DCMP,13) it is suggested that cardiac involvement of CSS may be considered as a reversible cause of DCMP, because DCMP in the present case was improved after 3 months of appropriate medical managements. To improve cardiac function in CSS patients with DCMP, the early identification of cardiac involvement by multi-modality imaging and appropriate medical therapy would be of importance.14) Considering the pathophysiologic mechanism of DCMP in patients with CSS, immunosuppressive therapy to control disease activity of CSS should be included, in addition to conventional medical therapy for heart failure including blocking agents for renin-angiotensin-aldosterone system and beta-blockade. The previous study have shown that high dose steroid and chemotherapy with cyclophosphamide are the most effective treatment of cardiac involvement of CSS, even though cyclophosphamide has a potential of cardiotoxicity.15)
In conclusion, the present case demonstrated that DCMP may develop as an unusual form of cardiac involvement in the course of CSS, and the DCMP associated with CSS may be reversible by appropriate medical managements including immunosuppressive therapy.
Figures and Tables
Fig. 1
Magnetic resonance image (MRI) findings of the brain. MRI image findings of the brain reveals multi-focal acute cerebral infarctions (indicated by white arrows).
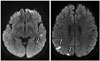
Fig. 2
Electrocardiogram findings. Electrocardiogram findings on admission (A) and at discharge (B).
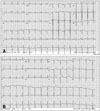
Fig. 3
Chest X-ray and echocardiographic findings. Chest X-ray and echocardiographic findings before (A-D) and after treatment (E-H). Cardiomegaly and pulmonary infiltrates on chest X-ray, and left ventricular (LV) dysfunction on echocardiography were improved after 3 months of medical therapy. Asterisk (*) indicated LV thrombi.
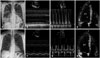
Fig. 4
Magnetic resonance image findings of the heart. Magnetic resonance imaging of the heart reveals multiple high signal intensity in subepicardial and subendocardial area of left ventricular myocardium with multifocal delayed enhancement (arrowheads). Consolidative lesions with high signal intensity in right middle lobe were also noted (asterisk).
References
1. Grau RG. Churg-Strauss syndrome: 2005-2008 update. Curr Rheumatol Rep. 2008; 10:453–458.
2. Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990; 33:1094–1100.
3. Solans R, Bosch JA, Pérez-Bocanegra C, Selva A, Huguet P, Alijotas J, Orriols R, Armadans L, Vilardell M. Churg-Strauss syndrome: outcome and long-term follow-up of 32 patients. Rheumatology (Oxford). 2001; 40:763–771.
4. Aakerøy L, Amundsen BH, Skomsvoll JF, Haugen BO, Soma J. A 50-year-old man with eosinophilia and cardiomyopathy: need for endomyocardial biopsy. Eur J Echocardiogr. 2011; 12:257–259.
5. Seo JS, Song JM, Kim DH, Kang DH, Song JK. A Case of Loeffler's Endocarditis Associated with Churg-Strauss Syndrome. J Cardiovasc Ultrasound. 2010; 18:21–24.
6. Wolf J, Bergner R, Mutallib S, Buggle F, Grau AJ. Neurologic complications of Churg-Strauss syndrome--a prospective monocentric study. Eur J Neurol. 2010; 17:582–588.
7. Abu-Shakra M, Smythe H, Lewtas J, Badley E, Weber D, Keystone E. Outcome of polyarteritis nodosa and Churg-Strauss syndrome. An analysis of twenty-five patients. Arthritis Rheum. 1994; 37:1798–1803.
8. Finsterer J. Neurological manifestations of Churg-Strauss syndrome. Eur J Neurol. 2010; 17:524–525.
9. Dennert RM, van Paassen P, Schalla S, Kuznetsova T, Alzand BS, Staessen JA, Velthuis S, Crijns HJ, Tervaert JW, Heymans S. Cardiac involvement in Churg-Strauss syndrome. Arthritis Rheum. 2010; 62:627–634.
10. Szczeklik W, Miszalski-Jamka T, Mastalerz L, Sokolowska B, Dropinski J, Banys R, Hor KN, Mazur W, Musial J. Multimodality assessment of cardiac involvement in Churg-Strauss syndrome patients in clinical remission. Circ J. 2011; 75:649–655.
11. Marmursztejn J, Vignaux O, Cohen P, Guilpain P, Pagnoux C, Gouya H, Mouthon L, Legmann P, Duboc D, Guillevin L. Impact of cardiac magnetic resonance imaging for assessment of Churg-Strauss syndrome: a cross-sectional study in 20 patients. Clin Exp Rheumatol. 2009; 27:1 Suppl 52. S70–S76.
12. Mavrogeni S, Manoussakis MN, Karagiorga TC, Douskou M, Panagiotakos D, Bournia V, Cokkinos DV, Moutsopoulos HM. Detection of coronary artery lesions and myocardial necrosis by magnetic resonance in systemic necrotizing vasculitides. Arthritis Rheum. 2009; 61:1121–1129.
13. Sliwa K, Damasceno A, Mayosi BM. Epidemiology and etiology of cardiomyopathy in Africa. Circulation. 2005; 112:3577–3583.
14. Park JH, Kwon DH, Starling RC, Marwick TH. Role of imaging in the detection of reversible cardiomyopathy. J Cardiovasc Ultrasound. 2013; 21:45–55.
15. Gharib MI, Burnett AK. Chemotherapy-induced cardiotoxicity: current practice and prospects of prophylaxis. Eur J Heart Fail. 2002; 4:235–242.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download