Abstract
Background
Currently there is no noninvasive imaging modality used to risk stratify patients requiring lead extractions. We report the novel use of superior vena cava (SVC) echocardiography to identify lead fibrosis and complex cardiac implantable electronic device (CIED) lead extraction. With an aging population and expanding indications for cardiac device implantation, the ability to deal with the complications associated with chronically implanted device has also increased.
Methods
This was a retrospective analysis of Doppler echocardiography recorded in our outpatient Electrophysiology/Device Clinic office over 6 months. Images from 109 consecutive patients were reviewed.
Results
62% (68/109) did not have a CIED and 38% (41/109) had a CIED. In patients without a CIED, 6% (4/68) displayed turbulent color flow by Doppler in the SVC, while 22% (9/41) of patients with a CIED displayed turbulent flow. Fisher's exact test found a statistically significant difference between the two groups (p value < 0.05). The CIED group was subdivided into 2 groups based on device implant duration (< 2 years vs. ≥ 2 years). Of the CIED implanted for ≥ 2 years, 27% (9/33) had turbulent flow in the SVC by Doppler, while no patients (0/8) with implant durations < 2 years demonstrated turbulent flow. Nine patients underwent subsequent lead extraction. A turbulent color pattern successfully identified all 3 patients that had significant fibrosis in the SVC found during extraction.
Over 3 million patients received a pacemaker or implantable cardioverter-defibrillators (ICD) in the United States between 1993 and 2006.1) With an aging population and expanding indications for cardiac device implantation, the ability to deal with the complications associated with a chronically implanted device has also increased. Approaches to deal with these complications have been debated, with removal of the device as a focus of investigation.2)3)
The Heart Rhythm Society has set guidelines for clinical practice describing when it is appropriate to consider cardiac implantable electronic device (CIED) removal.2)3) As with any invasive procedure, there are risks associated with cardiac lead extraction.4) Major adverse events reported to the Food and Drug Administration between 1995 and 2008 during ICD lead extractions showed the most common complications were laceration of the right atrium, superior vena cava (SVC), and the innominate vein.5) Although the percentage is low, these potential complications could necessitate emergent surgery or even result in death.4)5) Careful preoperative evaluation therefore needs to be considered.
Lead extractions with high-risk features, such as significant fibrosis adherent to the lead and vascular wall, require powered sheaths and tools such as laser sheaths to successfully remove the lead. The most common sites of fibrosis appear to be at the junction of the innominate vein and the SVC, at the right atrium/SVC junction, the tricuspid valve, and from the anode ring to the lead tip.5)6) Since there may be a correlation between degree of fibrosis and periprocedure morbidity and mortality, determining the degree of lead fibrosis could help stratify perioperative risk during lead extraction.
Intracardiac and transesophageal echocardiography have been used to evaluate leads prior to extraction. These modalities both have the potential to visualize scar tissue around leads but both are invasive and limited in their ability to view the SVC and innominate veins.7)8) Another option is venography. There have been studies to assess SVC thrombus and occlusion related to ICD and pacemaker leads.9)10)11) Venography could be helpful to assess lead fibrosis, but involves the obvious risk of intravenous contrast. To date there is no ideal noninvasive modality to evaluate the degree of lead associated fibrosis in the SVC.
We report a case series of transthoracic echocardiography (TTE) utilizing color Doppler to view the SVC in patients with CIEDs prior to lead extraction. Our observations suggest that significant lead fibrosis in the SVC causing turbulent flow patterns can be detected using transthoracic echocardiography with color Doppler.
The study was reviewed and approved by our Institutional Review Board. This was a retrospective analysis of Doppler echocardiography recorded in our outpatient Electrophysiology/Device Clinic office over 6 months. Images from 109 consecutive patients were reviewed. 62% (68/109) of the patients did not have a CIED, and 38% (41/109) patients had a device. Data from chart reviews included patient and lead demographics, indication for extraction, and fibrotic regions noted during time of extraction. All echocardiography studies included in this analysis had a 2 dimensional (2D) view of the innominate veins as they flow into the SVC and color flow Doppler with Nyquist Limits from 50 cm/sec to 70 cm/sec. One expert echocardiographer reviewed all the echoes, blinded to the patient and lead demographics. Excluded from the study results were any patients with congenital cardiac abnormalities.
The primary outcome of this study was the presence of a turbulent flow pattern by color Doppler. We used Fisher's exact test and p-value of less than 0.05 to determine statistical significance between the groups with and without a CIED (Table 1), and to assess for the presence or absence of turbulent flow in the SVC (Table 2). We then compared the incidence of turbulent flow in patients with device implant durations less than 2 years versus patients with implant durations greater than 2 years. Based on our clinical experience, we chose 2 years as the time point to define the implant durations as new versus chronically implanted. We compared turbulent flow with actual site of fibrosis directly visualized during the extraction procedure with fluoroscopy.
Of the 109 patients, 62% were without a CIED and 38% had a device. Basic patient demographics are shown in Table 1. We chose to include presence of pulmonary hypertension and tricuspid regurgitation in our analysis in order to control for possible confounding variables that may potentially influence laminar flow in the SVC. There was no statistically significant difference in these variables between the two groups.
Of the 68 echoes in patients who did not have a CIED, only 6% (4/68) of them displayed turbulent flow patterns in the SVC. Of the 41 patients who did have a device, 22% (9/41) had turbulence in the SVC (Table 2). Using Fisher exact test the p-value = 0.016.
Of the 41 patients with CIEDs, 9 underwent subsequent extraction. The most common indication for extraction was CIED infection (67%) followed by lead failure (11%) (Table 3). The frequency of these indications is consistent with the established literature.5) Of the 41 patients with CIEDs, only 8 devices had implant durations less than 2 years prior. None of these patients had turbulent flow patterns in the SVC. Of the patients with device implant durations greater than 2 years, 27% (9/33) of patients displayed turbulence in the SVC. Of the 41 patients with CIED, 9 patients had their lead(s) extracted. Of the nine patients that underwent lead extraction, 33% (3/9) had turbulent flow patterns in the SVC; all three patients had visible fibrosis in the SVC during the extraction procedure and required advanced tools, such as laser sheaths.
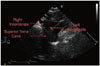
TTE is a simple, portable, and low risk procedure. As our study demonstrates, the supraclavicular view of the SVC may provide important clinical information prior to lead extraction. This view is easily reproducible, usually requiring only slight alterations in the scan plane horizontally or in the anterior/posterior plane to optimally visualize the SVC. Done correctly, this 2D view including color Doppler requires minimal additional effort and time. It allows visualization not only the top 4 cm of the SVC but also the right innominate vein, its formation by the right subclavian and internal jugular veins, and the left innominate vein (Fig. 1).12) Normal flow in the SVC is laminar and includes two large antegrade flow waves during ventricular systole and diastole. Under normal physiologic conditions these waves will increase in velocity during inspiration and decrease during exhalation.12)13)14)15)
A literature search for the use of transthoracic imaging of the SVC in the setting of pacemaker/ICD lead fibrosis yielded minimal results. One study examined severe stenosis caused by pacemaker leads in the SVC and innominate vein. SVC echocardiography using color and pulse wave Doppler was found to be a sensitive test when compared to venography for diagnosis of severe stenosis.14)
SVC flow has been examined in other clinical scenarios. In children with central lines, SVC echocardiography showed no change from baseline flow rates or degree of laminar flow compared to a SVC without central line. However, those with thrombus associated with the central line had more turbulent flow.15)
This study highlights the use of TTE of the SVC preoperatively for lead extraction. We found statistical significance (Table 2) comparing turbulent flow patterns between patients with a CIED and those without (Fig. 2). We compared the incidence of turbulence in new versus chronically implanted devices. Based on our own unpublished data and supported by the literature, device leads with longer implant durations are more likely to develop fibrosis and are more difficult to extract.16)17)18)19)20)21) Our echocardiography results in this study demonstrate that turbulent flow occurred more frequently in leads with implant durations greater than 2 years prior. Of the patients that underwent lead extraction, three patients had turbulent flow within the SVC. All three patients had fibrosis visualized within the SVC, using fluoroscopy, during extraction procedures requiring advanced tools, such as laser sheaths.
The limitation of our study is the small number of patients, especially in our subset of newly implanted devices and patient that underwent lead extraction. Though only 109 patients were included in this case series, 9 patients underwent lead extraction by a single operator, within the same window of time using the same extraction techniques. All 3 patients that had turbulent flow on screening SVC echocardiography required advanced extraction tools. The remaining 6 patients that underwent lead extraction, all had normal Doppler color flow patterns, without any turbulence, successfully predicting uncomplicated extraction procedures, requiring only simple traction for lead removal. This is the first case series reported in the literature to date, reviewing the clinical utility of using SVC echocardiography prior to lead extraction in predicting complex procedures.
If SVC echocardiography proves reproducible and sensitive for detection of significant SVC fibrosis, it could serve as a screening tool to guide clinical decision-making, and facilitate the referral of a patient with significant fibrosis to an experienced lead extraction center. Our study raises several clinically relevant questions. What is the true sensitivity and specificity of turbulent SVC flow by color Doppler in identifying significant lead fibrosis? Can the addition of pulse wave Doppler or continuous wave Doppler increase the sensitivity and specificity of identifying significant lead fibrosis with preoperative transthoracic echo? Can screening with TTE decrease procedure-related morbidity and mortality associated with CIED extraction?
Figures and Tables
Fig. 2
Color Doppler showing turbulent flow within the superior vena cava (SVC) (A) and displays normal flow in the SVC (B).

References
1. Kurtz SM, Ochoa JA, Lau E, Shkolnikov Y, Pavri BB, Frisch D, Greenspon AJ. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993-2006. Pacing Clin Electrophysiol. 2010; 33:705–711.
2. Wilkoff BL, Love CJ, Byrd CL, Bongiorni MG, Carrillo RG, Crossley GH 3rd, Epstein LM, Friedman RA, Kennergren CE, Mitkowski P, Schaerf RH, Wazni OM. Heart Rhythm Society. American Heart Association. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm. 2009; 6:1085–1104.
3. Love CJ, Wilkoff BL, Byrd CL, Belott PH, Brinker JA, Fearnot NE, Friedman RA, Furman S, Goode LB, Hayes DL, Kawanishi DT, Parsonnet V, Reiser C, Van Zandt HJ. Recommendations for extraction of chronically implanted transvenous pacing and defibrillator leads: indications, facilities, training. North American Society of Pacing and Electrophysiology Lead Extraction Conference Faculty. Pacing Clin Electrophysiol. 2000; 23(4 Pt 1):544–551.
4. Kutalek SP. Pacemaker and defibrillator lead extraction. Curr Opin Cardiol. 2004; 19:19–22.
5. Hauser RG, Katsiyiannis WT, Gornick CC, Almquist AK, Kallinen LM. Deaths and cardiovascular injuries due to device-assisted implantable cardioverter-defibrillator and pacemaker lead extraction. Europace. 2010; 12:395–401.
6. Smith HJ, Fearnot NE, Byrd CL. Where does scar tissue form to inhibit extraction of chronic pacemaker leads (Abstract). J Am Coll Cardiol. 1992; 19:148A.
7. Lo R, D'Anca M, Cohen T, Kerwin T. Incidence and prognosis of pacemaker lead-associated masses: a study of 1,569 transesophageal echocardiograms. J Invasive Cardiol. 2006; 18:599–601.
8. Bongiorni MG, Di Cori A, Soldati E, Zucchelli G, Arena G, Segreti L, De Lucia R, Marzilli M. Intracardiac echocardiography in patients with pacing and defibrillating leads: a feasibility study. Echocardiography. 2008; 25:632–638.
9. Bulur S, Vural A, Yazıcı M, Ertaş G, Özhan H, Ural D. Incidence and predictors of subclavian vein obstruction following biventricular device implantation. J Interv Card Electrophysiol. 2010; 29:199–202.
10. Korkeila P, Mustonen P, Koistinen J, Nyman K, Ylitalo A, Karjalainen P, Lund J, Airaksinen J. Clinical and laboratory risk factors of thrombotic complications after pacemaker implantation: a prospective study. Europace. 2010; 12:817–824.
11. Korkeila P, Ylitalo A, Koistinen J, Airaksinen KE. Progression of venous pathology after pacemaker and cardioverter-defibrillator implantation: a prospective serial venographic study. Ann Med. 2009; 41:216–223.
12. Khouzam RN, Minderman D, D'Cruz IA. Echocardiography of the superior vena cava. Clin Cardiol. 2005; 28:362–366.
13. Cohen ML, Cohen BS, Kronzon I, Lighty GW, Winer HE. Superior vena caval blood flow velocities in adults: a Doppler echocardiographic study. J Appl Physiol (1985). 1986; 61:215–219.
14. Nishino M, Tanouchi J, Ito T, Tanaka K, Aoyama T, Kitamura M, Nakagawa T, Kato J, Yamada Y. Echographic detection of latent severe thrombotic stenosis of the superior vena cava and innominate vein in patients with a pacemaker: integrated diagnosis using sonography, pulse Doppler, and color flow. Pacing Clin Electrophysiol. 1997; 20(4 Pt 1):946–952.
15. Hammerli M, Meyer RA. Doppler evaluation of central venous lines in the superior vena cava. J Pediatr. 1993; 122:S104–S108.
16. Kohut AR, Grammes J, Schulze CM, Al-Bataineh M, Yesenosky GA, Horrow JC, Kutalek SP. Percutaneous extraction of ePTFE-coated ICD leads: a single center comparative experience. Pacing Clin Electrophysiol. 2013; 36:444–450.
17. Maytin M, Epstein LM, John RM. Lead implant duration does not always predict ease of extraction: extraction sheath may be required at < 1 year. Pacing Clin Electrophysiol. 2011; 34:1615–1620.
18. Jones SO 4th, Eckart RE, Albert CM, Epstein LM. Large, single-center, single-operator experience with transvenous lead extraction: outcomes and changing indications. Heart Rhythm. 2008; 5:520–525.
19. Farooqi FM, Talsania S, Hamid S, Rinaldi CA. Extraction of cardiac rhythm devices: indications, techniques and outcomes for the removal of pacemaker and defibrillator leads. Int J Clin Pract. 2010; 64:1140–1147.
20. Smith MC, Love CJ. Extraction of transvenous pacing and ICD leads. Pacing Clin Electrophysiol. 2008; 31:736–752.
21. Bracke F, Meijer A, Van Gelder B. Extraction of pacemaker and implantable cardioverter defibrillator leads: patient and lead characteristics in relation to the requirement of extraction tools. Pacing Clin Electrophysiol. 2002; 25:1037–1040.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download