1. Cruz ML, Goran MI. The metabolic syndrome in children and adolescents. Curr Diab Rep. 2004; 4:53–62.

2. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003; 157:821–827.

3. Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, Mietus-Snyder ML. American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young. Council on Cardiovascular Nursing. and Council on Nutrition, Physical Activity, and Metabolism. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009; 119:628–647.

4. Ford ES, Li C. Defining the metabolic syndrome in children and adolescents: will the real definition please stand up? J Pediatr. 2008; 152:160–164.

5. Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S. IDF Consensus Group. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007; 8:299–306.

6. Chen W, Srinivasan SR, Elkasabany A, Berenson GS. Cardiovascular risk factors clustering features of insulin resistance syndrome (Syndrome X) in a biracial (Black-White) population of children, adolescents, and young adults: the Bogalusa Heart Study. Am J Epidemiol. 1999; 150:667–674.

7. Kibar AE, Pac FA, Ballı S, Oflaz MB, Ece I, Bas VN, Aycan Z. Early subclinical left-ventricular dysfunction in obese nonhypertensive children: a tissue Doppler imaging study. Pediatr Cardiol. 2013; 34:1482–1490.

8. Shah N, Chintala K, Aggarwal S. Electrocardiographic strain pattern in children with left ventricular hypertrophy: a marker of ventricular dysfunction. Pediatr Cardiol. 2010; 31:800–806.

9. Lorch SM, Sharkey A. Myocardial velocity, strain, and strain rate abnormalities in healthy obese children. J Cardiometab Syndr. 2007; 2:30–34.

10. Movahed MR, Saito Y. Obesity is associated with left atrial enlargement, E/A reversal and left ventricular hypertrophy. Exp Clin Cardiol. 2008; 13:89–91.
11. Swaminathan S, Ferrer PL, Wolff GS, Gómez-MarÍn O, Rusconi PG. Usefulness of tissue Doppler echocardiography for evaluating ventricular function in children without heart disease. Am J Cardiol. 2003; 91:570–574.

12. Willens HJ, Chakko SC, Lowery MH, Byers P, Labrador E, Gallagher A, Castrillon JC, Myerburg RJ. Tissue Doppler imaging of the right and left ventricle in severe obesity (body mass index >35 kg/m
2). Am J Cardiol. 2004; 94:1087–1090.

13. Voigt JU, Arnold MF, Karlsson M, Hübbert L, Kukulski T, Hatle L, Sutherland GR. Assessment of regional longitudinal myocardial strain rate derived from Doppler myocardial imaging indexes in normal and infarcted myocardium. J Am Soc Echocardiogr. 2000; 13:588–598.

14. Voigt JU, Lindenmeier G, Exner B, Regenfus M, Werner D, Reulbach U, Nixdorff U, Flachskampf FA, Daniel WG. Incidence and characteristics of segmental postsystolic longitudinal shortening in normal, acutely ischemic, and scarred myocardium. J Am Soc Echocardiogr. 2003; 16:415–423.

15. Yuda S, Short L, Leano R, Marwick TH. Myocardial abnormalities in hypertensive patients with normal and abnormal left ventricular filling: a study of ultrasound tissue characterization and strain. Clin Sci (Lond). 2002; 103:283–293.

16. Gong HP, Tan HW, Fang NN, Song T, Li SH, Zhong M, Zhang W, Zhang Y. Impaired left ventricular systolic and diastolic function in patients with metabolic syndrome as assessed by strain and strain rate imaging. Diabetes Res Clin Pract. 2009; 83:300–307.

17. Grandi AM, Maresca AM, Giudici E, Laurita E, Marchesi C, Solbiati F, Nicolini E, Guasti L, Venco A. Metabolic syndrome and morphofunctional characteristics of the left ventricle in clinically hypertensive nondiabetic subjects. Am J Hypertens. 2006; 19:199–205.

18. Masugata H, Senda S, Goda F, Yoshihara Y, Yoshikawa K, Fujita N, Daikuhara H, Nakamura H, Taoka T, Kohno M. Left ventricular diastolic dysfunction as assessed by echocardiography in metabolic syndrome. Hypertens Res. 2006; 29:897–903.

19. Wong CY, O'Moore-Sullivan T, Fang ZY, Haluska B, Leano R, Marwick TH. Myocardial and vascular dysfunction and exercise capacity in the metabolic syndrome. Am J Cardiol. 2005; 96:1686–1691.

20. Bokor S, Frelut ML, Vania A, Hadjiathanasiou CG, Anastasakou M, Malecka-Tendera E, Matusik P, Molnár D. Prevalence of metabolic syndrome in European obese children. Int J Pediatr Obes. 2008; 3:Suppl 2. 3–8.

21. Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013; 62:1309–1319.
22. Nakajima T, Fujioka S, Tokunaga K, Matsuzawa Y, Tarui S. Correlation of intraabdominal fat accumulation and left ventricular performance in obesity. Am J Cardiol. 1989; 64:369–373.

23. Alpert MA, Lambert CR, Terry BE, Cohen MV, Mukerji V, Massey CV, Hashimi MW, Panayiotou H. Influence of left ventricular mass on left ventricular diastolic filling in normotensive morbid obesity. Am Heart J. 1995; 130:1068–1073.

24. Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003; 52:1779–1785.
25. Di Bello V, Giampietro O, Pedrinelli R, Matteucci E, Giorgi D, Bertini A, Bianchi M, Ferdeghini M, Boldrini E, Dell'Omo G, Paterni M, Giusti C. Can insulin action induce myocardial texture alterations in essential hypertension? Am J Hypertens. 1999; 12:283–290.

26. Mizushige K, Yao L, Noma T, Kiyomoto H, Yu Y, Hosomi N, Ohmori K, Matsuo H. Alteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin-resistant prediabetic stage of a type II diabetic rat model. Circulation. 2000; 101:899–907.

27. Vasan RS. Cardiac function and obesity. Heart. 2003; 89:1127–1129.

28. Dhuper S, Abdullah RA, Weichbrod L, Mahdi E, Cohen HW. Association of obesity and hypertension with left ventricular geometry and function in children and adolescents. Obesity (Silver Spring). 2011; 19:128–133.

29. Mahfouz RA, Dewedar A, Abdelmoneim A, Hossien EM. Aortic and pulmonary artery stiffness and cardiac function in children at risk for obesity. Echocardiography. 2012; 29:984–990.

30. Ingul CB, Tjonna AE, Stolen TO, Stoylen A, Wisloff U. Impaired cardiac function among obese adolescents: effect of aerobic interval training. Arch Pediatr Adolesc Med. 2010; 164:852–859.
31. Schuster I, Karpoff L, Perez-Martin A, Oudot C, Startun A, Rubini M, Obert P, Vinet A. Cardiac function during exercise in obese prepubertal boys: effect of degree of obesity. Obesity (Silver Spring). 2009; 17:1878–1883.

32. Burchfiel CM, Skelton TN, Andrew ME, Garrison RJ, Arnett DK, Jones DW, Taylor HA Jr. Metabolic syndrome and echocardiographic left ventricular mass in blacks: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2005; 112:819–827.

33. Iacobellis G, Pellicelli AM, Sharma AM, Grisorio B, Barbarini G, Barbaro G. Relation of subepicardial adipose tissue to carotid intima-media thickness in patients with human immunodeficiency virus. Am J Cardiol. 2007; 99:1470–1472.

34. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000; 102:1788–1794.

35. Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, Tian G, Kirkpatrick ID, Singal PK, Krahn M, Grenier D, Jassal DS. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011; 57:2263–2270.

36. Lago F, Gómez R, Gómez-Reino JJ, Dieguez C, Gualillo O. Adipokines as novel modulators of lipid metabolism. Trends Biochem Sci. 2009; 34:500–510.

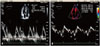
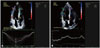
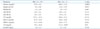
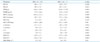






 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download