Introduction
Bicuspid aortic valve (BAV) is the most common congenital heart disease with an estimated prevalence of 0.5-2%.1)2)3) BAV is more commonly observed in male with approximate predominance of 3:1. Although BAV is a congenital anomaly, development of its complications in adulthood is a characteristic feature of the natural history of this disease entity. Moreover, morphological or functional abnormality associated with BAV is not confined to the aortic valve and development of complications in the adjacent aorta and other anatomical cardiac structures are not uncommon.4)5) Thus, thorough understanding of the complex clinical features of patients with BAV is not easy and there are many unresolved issues.
BAV Phenotype and Valvulopathy
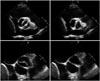
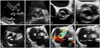
Normal aortic valve has 3 cusps with similar size separated by 3 commissures. BAV is typically made of 2 unequal-sized cusps with fusion of one commissure, which results in a central raphe or ridge in the larger cusp (Fig. 1). The phenotype or anatomical patterns of BAV can vary according to which commissures have fused. The classification of BAV phenotype has remained a challenging issue and needs standardization. Traditionally, classification based on fused two cusps and the orientation of the raphe has been used:6)7) for example, fusion of the right and left coronary cusps is classified as BAV-RL type (Fig. 2A). Thus, BAV-RN (BAV with fusion of the right and non-coronary cusps), and BAV-LN (BAV with fusion of the left and non-coronary cusps) based on the fused two cusps were suggested by some investigators. Although this classification seems logical, demonstration of fused 2 cusps is not easy or practical and sometimes the bicuspid cusps are symmetrical without raphe ("pure" BAV), which makes demonstration of fused 2 cusps impossible. For this circumstance, the traditional approach was to use the orientation of the free edge of the cusps defined either anterior-posterior (BAV-AP) or lateral (BAV-LA) (Fig. 2A). To overcome potential problems using two different criteria for BAV phenotypic classification (the orientation of the raphe and the free edge), a new classification has been suggested using the orientation of BAV only. In this classification,8) two BAV phenotypes-fusion of the right and left coronary cusps (BAV-AP) and fusion of the right or left coronary cusp and non-coronary cusp (BAV-RL)-were used (Fig. 2B). The position of both coronary artery ostia is also different according to the BAV phenotype (BAV-AP vs. BAV-RL), which is helpful for patients with relatively poor echocardiographic window. The other notable finding is that a recent animal experiment provided strong evidence that BAV-AP and BAV-RL types are distinct etiologic entities with the defective development of different embryological structures at different embryological time.9) The theoretical advantages of the new classification need to be tested by clinical outcome studies. Considering that a consistent description of BAV phenotype is absolutely necessary for assessment of its potential clinical or prognostic implication, a uniform classification scheme should be determined based on long-term follow-up data.
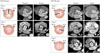
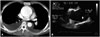
BAV phenotypic classification is attractive as it may provide new and valuable data regarding risk stratification of BAV patients according to cusp morphology. Of particular interest is the potential utility of this information, combined with knowledge of family history and hemodynamics, to provide a better understanding of patient prognosis. For that purpose, accurate imaging diagnosis of BAV is very important. It is no doubt that transthoracic echocardiography plays a pivotal role for diagnosis of BAV.5)6) However, not infrequently, other imaging modalities are necessary for correct diagnosis and classification of BAV. Sometimes, transesophageal echocardiography with better resolution is necessary for accurate diagnosis of BAV (Fig. 3). Special care is needed to identify the cusps separation and the site of cusp fusion. Both systolic and diastolic images are necessary for this purpose, as BAV might show a false tricuspid appearance in diastole based on the presence of the raphe. Frequently, the two commissures can be visualized only during systole with a clear separation of the cusps and thus it is imperative to pay particular attention to the opening motion of the aortic cusps (Fig. 1). In limited cases with heavy calcification, advanced imaging techniques such as computed tomography or cardiac magnetic resonance imaging would be the best option (Fig. 4).
Not all patients with BAV develop significant valvular dysfunction. Moreover, valvular dysfunction in BAV patients does show wide clinical spectrum in terms of patterns of valvular dysfunction; some patients show significant aortic stenosis, whereas others may show a prolapsing aortic cusp resulting in significant valvular regurgitation without stenotic component. The other extreme clinical spectrum includes development of both stenosis and regurgitation. Potential association between BAV phenotype and types of valvular dysfunction remains controversial and elusive. In young children and adolescents, BAV-RL type is reported to be associated with a higher risk of significant valvular dysfunction and more rapid development of aortic stenosis or aortic regurgitation with shorter time of intervention,10)11) which was not confirmed in adult patients with BAV.6)7) In a recent clinical study using high quality multidetector computed tomography, moderate-to-severe aortic stenosis predominated in patients with BAV-RL type, whereas moderate-to-severe aortic regurgitation in those with BAV-AP type;8) this potential association can be an interesting hypothesis which should be tested. All these studies suffer from selection bias characterized by relatively small numbers of patients with limited inclusion criteria such as referral for consideration of open heart surgery. Thus, potential association between specific BAV phenotype and types of valvular dysfunction still remains elusive and to be a hypothesis, which definitely needs further investigation.
BAV Aortopathy
Non-valvular cardiovascular conditions associated with BAV include coarctation of aorta, Turner syndrome, coronary artery anomalies, sinus of Valsalva aneurysm, aortic aneurysm, aortic dissection, supravalvular aortic stenosis, patent ductus arteriosus, ventricular septal defect, Shone's complex, and familial thoracic aortic aneurysm/dissection syndromes.12) Among them, aortic dilatation with development of aortic dissection is the most important and challenging issue in clinical practice. Common association of aortic dilatation with significant valvular dysfunction (aortic stenosis or regurgitation) in patients with BAV remains an important mechanism of development of aortic dissection.13)14) However, it is very interesting to observe that development of aortic enlargement or dissection is not correlated with severity of valvular dysfunction (Fig. 5) and can occur in BAV patients without valvular dysfunction or even several years after surgical correction of valvular dysfunction (Fig. 6).15)16)17)18)19) These findings support inherent primary abnormalities of the aortic media, more specifically cystic medial degeneration, a well-known risk factor associated with aortic aneurysm or dissection. In large family studies, the prevalence of BAVs in first degree relatives of an individual with a BAV has been reported to be 9% with autosomal dominance pattern20)21) and BAV associated with ascending aortic aneurysm may also be familial. Moreover, recent advances in molecular biology have demonstrated potential association with specific gene abnormalities, such as NOTCH1 (Notch homolog 1, translocation-associated) or transforming growth factor beta receptor.22)23)24) All these findings support a genetically triggered intrinsic defect involving the aortic media. However, in a recent cross-sectional analysis of 595 patients with BAV, age and severity of valvular dysfunction were proven to be two independent factors associated with aortic diameter. 14) Additionally, isolated aortic valve replacement for BAV patients with significant valvular dysfunction showed significantly lower annual aortic dilatation rate.14)25) These observations suggest significant impact of chronic hemodynamic burden due to valvular dysfunction and protective effect of surgical intervention. Further prospective investigations are necessary to overcome potential selection biases in the current clinical studies and to confirm factors associated with aortic dilatation in BAV patients.
Another characteristic finding of BAV aortopathy is that there are marked individual variations in terms of the site of aortic enlargement. Echocardiography has been the primary diagnostic tool for evaluation of BAV patients, which cannot evaluate the whole ascending and descending aorta. By using computer-assisted cluster analysis of clinical data of new imaging modalities, such as computed tomography or magnetic resonance imaging, various patterns of BAV aortopathy have been confirmed (Fig. 7).8)26) Aortic dilatation may confine to the aortic root, but, not infrequently, the aortic enlargement involves the tubular portion of the ascending aorta or entire ascending aorta up to the transverse aortic arch. Standardization of the classification of bicuspid aortopathy phenotypes is necessary for further investigation to test whether there would be potential association between BAV phenotype or type of valvular dysfunction with BAV aortopathy phenotypes.27) An interesting observation is that type of valvular dysfunction may explain different patterns of aortic dilatation in BAV patients: aortic regurgitation severity was found to be associated with aortic sinus diameter, whereas aortic stenosis severity with the tubular diameter.14)18) The association between BAV-RL type and aortic enlargement has been also reported and type 3 aortopathy, the most severe form of BAV aortopathy, was the most common phenotype in BAV-RL patients.8) As BAV-RL type showed higher frequency of moderate-to-severe aortic stenosis with a higher maximal transvalvular gradient or velocity, it may suggest that the hemodynamic burden contributes significantly to the development of bicuspid aortopathy. However, because progressive aortic dilatation occurs in many patients with normal valvular function, the hemodynamic burden caused by valvular dysfunction cannot entirely explain the full spectrum of bicuspid aortopathy. One interesting point is that the direction of helical flow differs according to BAV phenotype, with right-handed helical flow as the predominant pattern in BAV-AP type, and left-handed helical flow in BAV-RL type.28)29) Thus, variations in segmental aortic dilation that correlate with specific changes in valve morphology may be related to differences in eccentric flow jets that lead to a differential distribution of wall stress. Longitudinal follow-up is needed to determine whether an abnormal helical flow pattern correlates with BAV aortopathy phenotype. For this purpose, accurate classification of BAV aortopathy phenotypes is mandatory.
Natural History
Although BAV is characterized by a relatively long latent period before clinical manifestations in adulthood and diverse patterns of manifestations including valvular dysfunction (stenosis or regurgitation), aortopathy (aortic dissection), and acquired complications (infective endocarditis), the late outcomes in children with BAV, but without valve dysfunction, have not been well studied.4) Moreover, the earlier studies were from the era of cardiac catheterization. There are two large recent series to better define the un-operated natural history of BAV patients in the modern era.30)31)
The report from the Mayo clinic included 212 BAV patients without valvular dysfunction (mean age 32 ± 20 years) and the follow-up duration were 15 ± 6 years.30) Overall survival was excellent with the 20-year survival rate of 90 ± 3% and nobody died of cardiac diseases. The probability of aortic valve or aorta surgery was 27 ± 4% and no one underwent aortic dissection surgery. The incidence of endocarditis was 2%. Toronto general hospital group included 642 patients with a spectrum of valve dysfunction (mean age 35 ± 16 years) and the follow-up duration was 9 ± 5 years. The 10 year survival rate was 96 ± 1% and the probability of aortic valve or aorta surgery was 22 ± 2%. The incidence of aortic dissection and infective endocarditis were 2 ± 1% and 2%, respectively.31) These data support the notion held by many that eventually most patients with BAV would require some form of intervention. However, importantly, in both of these series, fatal events were rare and development of aortic dissection, the most serious clinical manifestation of BAV, does not occur frequently contrary to the previous expectation. Both studies confirmed that age and valvular dysfunction are independent risk factors associated with development of adverse clinical events in BAV patients. Thus, the risk of BAV patients should not be over-emphasized and the clinicians should encourage the patients and their family members to accept that the over-all prognosis of BAV patients is excellent with regular medical examination and follow-up in the modern era. Low incidence of aortic dissection in these series has been re-confirmed by another registry data showing 0.5% at 25 years follow-up13) and raised a challenging clinical issue of adequacy of current preventive or early surgical intervention for patients with BAV and aortic dilatation.32)33)34) However, a mere lack of data is responsible for the current controversy surrounding the indication and timing of elective surgical intervention for the aorta in BAV patients. It is obvious that aortic size and evidence of medial degeneration are limited risk stratification tools. A collaborative multicenter retrospective and prospective BAV patient registry with homogeneous and rigorous entry criteria (i.e., accurate BAV diagnosis and phenotyping with exclusion of unclear cases) is absolutely necessary to reconcile the clinical, imaging, pathobiology, genetic, and management pieces of the puzzle.5)
Conclusions
BAV patients show marked heterogeneity in many different clinical aspects including BAV phenotype, severity of valvular dysfunction and BAV aortopathy phenotype. Despite recent advances in clinical cardiology, many challenging issues still remain unresolved and elusive. Risk stratification based on imaging data combined with knowledge of genetics, family history and hemodynamics should be a clinicians' final goal. A well-characterized BAV cohort should be established and followed over time to achieve that goal. The current effort to establish a prospective Korean registry of BAV should be encouraged to get the fundamental data for evidence-based medical practice. Phenotype classification of BAV and BAV aortopathy based on high quality imaging studies is a key for BAV cohort and registry,35)36) which reinforces the leading role of image specialists for this project.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




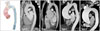
 XML Download
XML Download