Abstract
The differential diagnosis of cardiac mass is important in determining the therapeutic plan and avoiding unnecessary surgical intervention. Non-invasive imaging methods would be useful in the diagnosis of suspected cardiac mass, because they may provide earlier diagnosis and more accurate assessment of cardiac mass. Native aortic valve thrombosis is a rare disorder and difficult to differentiate from a tumor, and in particular, a papillary fibroelastoma. Thus, the clinical decision making with imaging modalities should be performed cautiously. We recently met a female patient who had a aortic valve mass resembling papillary fibroelastoma in normal native valve. The patient underwent a surgical resection and the pathologic finding showed an organized thrombus with no evidence of papillary fibroelastoma.
The differential diagnosis of cardiac masses is important in determining the therapeutic plan. Imaging characteristics are usually helpful in the differentiation of various cardiac masses with high probability. The differential diagnosis of aortic thrombus like tumor and vegetation is crucial for avoiding unnecessary surgical intervention. Non-invasive imaging methods would be useful in the diagnosis of suspected cardiac mass, because they may provide earlier diagnosis and more accurate assessment of cardiac mass. However, the clinical decision making with imaging modalities should be performed cautiously. We recently met a patient who had a native aortic valve thrombosis mimicking papillary fibroelastoma.
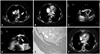
A 57-year-old female was referred to our hospital because of dyspnea worsening for 2 days. She had no hypertension, diabetes, or prior spontaneous abortion. At presentation, physical examination revealed that arterial pressure was 140/90 mmHg, heart rate was 110 beats/min, and respiration was 20 breaths/min. Mild pitting edema was noted in the right lower extremities. She was found to have both a lupus anticoagulant and antibodies to beta2-glycoprotein I of IgG, type. IgG and IgM anticardiolipin antibodies, antinuclear antibodies, double-stranded DNA antibodies, and venereal disease research laboratory tests were negative. Procoagulant work up including protein C, protein S, antithrombin, urine homocystine assays were normal. Factor V leiden mutation was not seen. The twelve lead electrocardiogram demonstrated sinus tachycardia and non-specific ST-T segments changes in precordial leads. A chest radiography showed mild cardiomegaly (cardiothoracic ratio, 0.55). Color Doppler and duplex-scan ultrasonography of the lower limbs presented deep venous thrombosis in right common femoral vein, superficial femoral vein, and popliteal vein. Transthoracic echocardiography (TTE) showed a dilated right ventricle associated with severely depressed systolic function (Supplementary movie 1 and 2). Computed tomography (CT) with contrast confirmed a diagnosis of pulmonary embolism via the disclosure of clots at the bifurcation of the common pulmonary artery and in its main branches (Fig. 1A). In additions, the aortic valve mass, located at the non-coronary cusp, resembling papillary fibroelastoma was discovered incidentally during chest CT (Fig. 1B). Its margin was smooth, and internal contour was homogeneous comparatively. However, the aortic mass was not diagnosed initially with TTE due to poor acoustic windows. Four hours later, her vital signs were unstable; heart rate was 110 beats/min, blood pressure was 90/60 mmHg, and she was breathing at a rate of 26/min. Because of persistent right ventricular dysfunction and progressive clinical deterioration, thrombolytic therapy were immediately performed. After initial treatments, a repeat echocardiography the following day revealed clear improvement in the right ventricular (RV) dysfunction with normal RV dimension. Subsequently, a transesophageal echocardiogram (TEE) was performed for evaluation of the aortic valve, revealing a 1 cm sized mobile cylindrical mass in the aortic valve. It attached without stalk to the non-coronary cusp annulus of the aortic valve and to extend into the ascending aorta. The aortic valve was tricuspid, without dystrophic calcification, and had no functional abnormality. In addition, there was no intracardiac shunt such as patent ductus arteriosus or atrial septal defect. The aortic valve mass was initially considered as a thrombus according to the presence of deep vein thrombosis and pulmonary embolism. After 2 weeks anticoagulation, however, a repeat TEE revealed that there was still a mass over 1 cm long at the non-coronary cusp of the aortic valve (Fig. 1C and D, Supplementary movie 3 and 4). Because a size change of the mass was not found after 2 weeks anticoagulation, we concluded that the aortic valve mass was thought to be a papillary fibroelastoma rather than thrombus.1) Therefore, surgical resection was considered as the best treatment option in this patient with mobile mass to prevent fatal embolic complications and to confirm pathologic findings. The following day, operation was performed under cardiopulmonary bypass. Aortic valve mass was easily removed and the valve was preserved. Gross inspection of cardiac mass showed a red-colored gelatinous lobulated mass measuring 1.4 × 0.9 cm. In the surgeon's opinion, the aortic mass was suggested as a diagnosis of organized thrombus. The histology of specimen revealed an organized thrombus with no evidence of papillary fibroelastoma (Fig. 1E).
Postoperative CT showed complete removal of the thrombus (Fig. 1F). She was diagnosed with antiphospholipid antibody syndrome based on her clinical presentation and laboratory findings. We repeated the lab tests after 12 weeks, which confirmed the diagnosis of antiphospholipid antibody syndrome.
Thrombosis in a native aortic valve is rarely reported and may result in clinically severe complications such as acute myocardial infarction, peripheral ischemia, cardiogenic shock and sudden death.2)3) Native aortic valve thrombosis may originate in heart valve disease, a hypercoagulative state or an auto-immune disease.4)5) Antiphospholipid antibody syndrome (APS) is an important cause of thromboembolic disease and fetal loss in the general population. Cardiac manifestations in the APS include valvulopathy, coronary artery disease, intracardiac thrombus and pulmonary hypertension.6) However, intracardiac thrombus is a rare complication of the APS and can occur in any cardiac chamber.7) Recent study have reported that the prevalence of intracardiac thrombus was less than 5% in the APS.8)
Differential diagnosis of aortic thrombus like tumor and vegetation is crucial for avoiding unnecessary surgical intervention. Non-invasive imaging methods would be useful in the diagnosis of suspected cardiac mass, because they may provide earlier diagnosis and more accurate assessment of cardiac mass. Recently, advanced imaging techniques improve the sensitivity and specificity.9) However, it is usually difficult to differentiate aortic thrombus from tumors, and especially papillary fibroelastoma which is the most common valvular tumor. Most papillary fibroelastomas are asymptomatic, but the lesions are recognized as a cause of embolisms. Diagnosis is accomplished incidentally by echocardiography that is usually performed for another purpose. Echocardiogram usually demonstrates a small, mobile, pedunculated or sessile valvular or endocardial mass, which on many occasions flutters or prolapses into the cardiac chambers during systole or diastole.1) As the tumors are small and attached to moving valves, they are usually not seen on CT or magnetic resonance images. The differential diagnosis of a papillary fibroelastoma includes other cardiac tumors, thrombus and vegetations. The tumors are rarely found on valves. Thrombus can be differentiated by an irregular or lobulated shape, laminated apperance, microcavitations and absence of a pedicle.10)
Although imaging tools are continually being developed, they may be clinically challenging to diagnose a cardiac mass. This case highlights the limitation of imaging tools for cardiac mass to lead a diagnosis. Even though, in the presence of the characteristic imaging, clinical decision making with imaging modalities should be performed cautiously.
Figures and Tables
Fig. 1
A: Chest computed tomography with contrast showing pulmonary embolism at the bifurcation of the common pulmonary artery and in its main branches (arrow). B: Computed tomography showing a filling defect attached to aortic valve (arrow). C: Short-axis view of transesophageal echocardiogram showing mass filling the non-coronary sinus of Valsalva (arrow). D: Long-axis view of transesophageal echocardiogram showing a mass just behind the non-coronary cusp (arrow). E: Histologic photomicrography of cardiac mass showing organized thrombus (hematoxylin & eosin stain, × 40). F: Follow-up computed tomography after surgical excision showing complete disappearance of the thrombus.
References
1. Sun JP, Asher CR, Yang XS, Cheng GG, Scalia GM, Massed AG, Griffin BP, Ratliff NB, Stewart WJ, Thomas JD. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation. 2001; 103:2687–2693.

2. Yamawaki M, Shimoyama M, Furuse Y, Kinugasa Y, Ogino K, Enokida M, Teshima R, Kitamura Y, Hayashi K, Shigemasa C. Floating thrombus arising from left sinus of Valsalva induced intermittent occlusion of left coronary artery and caused cardiogenic shock. Int J Cardiol. 2007; 114:272–273.

3. Nagata Y, Miyamoto T, Komura M, Niwa A, Kawaguchi S, Shirai T, Fujiwara H, Isobe M. Giant organized thrombus in the left sinus of valsalva causing intermittent left coronary obstruction: an unusual case of acute myocardial infarction. Circ J. 2004; 68:795–798.

4. Cho SJ, Yang JH, Shin JU, Uhm JE, Lee SC, Park SW, Park PW. A case of spontaneous native aortic valvular thrombosis that caused aortic stenoinsufficiency in the bicuspid aortic valve. Korean Circ J. 2006; 36:666–668.

5. Grondin F, Giannoccaro JP. Antiphospholipid antibody syndrome associated with large aortic valve vegetation and stroke. Can J Cardiol. 1995; 11:133–135.
6. Hegde VA, Vivas Y, Shah H, Haybron D, Srinivasan V, Dua A, Gradman A. Cardiovascular surgical outcomes in patients with the antiphospholipid syndrome--a case-series. Heart Lung Circ. 2007; 16:423–427.

7. Weiss S, Nyzio JB, Cines D, Detre J, Milas BL, Narula N, Floyd TF. Antiphospholipid syndrome: intraoperative and postoperative anticoagulation in cardiac surgery. J Cardiothorac Vasc Anesth. 2008; 22:735–739.

8. Djokovic A, Stojanovich L, Kontic M, Stanisavljevic N, Radovanovic S, Marisavljevic D. Association between cardiac manifestations and antiphospholipid antibody type and level in a cohort of Serbian patients with primary and secondary antiphospholipid syndrome. Isr Med Assoc J. 2014; 16:162–167.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download