Abstract
Recently, transcatheter aortic valve replacement (TAVR) has emerged as an alternative for the treatment of severe symptomatic aortic stenosis patients. Although experience with TAVR is increasing exponentially, few cases of post-TAVR endocarditis are reported. We present a case of 76-year-old man with infective endocarditis after TAVR who was definitely diagnosed by echocardiography.
Transcatheter aortic valve replacement (TAVR) is currently an alternative to surgical aortic valve replacement (SAVR) in high surgical risk patients with severe aortic stenosis (AS).1) Although the experience of TAVR is increasing in the medical communities worldwide, few cases of infective endocarditis (IE) after TAVR has been reported. According to previous studies, IE after TAVR ranges between 0% and 2.3%.2) At the current status, there are no clear guidelines as to how to diagnose or how to treat this complication,2) however nearly all of the patients undergo a very high-risk salvage surgical procedure for IE. Furthermore, the unique mechanism leading to IE after TAVR is largely unknown. Here we report a patient with IE after TAVR with some unique features that need discussion.
A 76-year-old male (preoperative logistic EuroSCORE 9.92%) underwent an uneventful TAVR with a 29 mm CoreValve for severe symptomatic AS in January 2012 (Fig. 1 and 2, Supplementary movie 1, 2, 3, 4). He had hypertension and atrial fibrillation. He also had a VVI pacemaker implanted for sick sinus syndrome in 2002. Furthermore, he suffered from ischemic heart disease which had been treated by percutaneous coronary intervention to mid-left anterior descending coronary artery 7 years before. Although the patient's logistic EuroSCORE was relatively low, he and his family strongly preferred TAVR than SAVR. Before the procedure, transthoracic echocardiography demonstrated severe AS (peak/mean gradient 90/55 mmHg, aortic valve peak velocity 4.74 m/s, aortic valve area 0.73 cm2 by continuity equation) with normal left ventricle (LV) function (ejection fraction 69%) and there was no pulmonary hypertension. He was discharged 7 days after the procedure with only mild paravalvular aortic regurgitation (AR).
He was readmitted 2 months after TAVR for fever, dyspnea and mild confusion. On meticulous history taking, he had periodontitis, for which incision and drainage of the abscess was performed 1 month prior to admission at a nearby hospital.
Inflammatory markers including white blood cell count (19700/mm3) and C-reactive protein level (11.54 mg/dL) were markedly elevated. Simple chest X-ray revealed bilateral pleural effusion (Fig. 3A) and electrocardiogram showed atrial fibrillation with rapid ventricular response and left bundle branch block (Fig. 3B). Transthoracic echocardiography showed a normal sized LV with normal systolic function. However, severe mitral regurgitation was noted just beneath the strut of the bioprosthesis (Fig. 4A, Supplementary movie 5). Transesophageal echocardiography was performed without delay, which demonstrated a large mobile vegetation attached to the anterior mitral leaflet and severe mitral regurgitation due to multiple perforations of the mitral valve leaflet. Abscess was also noted at the aortomitral continuity (Fig. 4B, Supplementary movie 6). The bioprosthetic aortic valve function was normal.
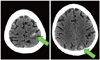
We performed brain imaging studies for evaluation of altered mentality. Both intracerebral and subarachnoid hemorrhage was detected on brain computed tomography imaging (Fig. 5). Vancomycin, gentamicin and rifampin were started without delay under the diagnosis of IE. Streptococcus anginosus was identified on all three separate blood cultures.
The patient and his family members refused surgical intervention because of the high risk. The patient died 4 days after hospital admission due to refractory sepsis. The autopsy was refused.
This case is noteworthy for a few reasons. First of all, the location of the implanted device and the paravalvular leakage may be a possible 'local' risk factor for IE after TAVR. In this case, the site of mitral regurgitation after IE was just located beneath the strut of the aortic bioprosthesis. Therefore, although the device was implanted adequately according to the manufacturer's recommendation, repetitive mechanical irritation by the bioprosthesis strut might have been a risk factor for IE.3) Meanwhile some degree of paravalvular leak is common after TAVR4) and considering that paravalvular leakage is a known risk factor for IE after TAVR, the space between the bioprosthesis and the native aortic valve cusp might be a suitable nidus for pathogen accumulation during transient bacteremia.5) This case of our patient also demonstrated paravalvular leak associated with the aortic valve bioprosthesis. Collectively, both paravalvular AR and irritation of the mitral valve by the stent struts may have equally contributed to endothelial damage and subsequent IE.
One might also suggest that migration of the bioprosthesis may have contributed to the devastating result in our patient. Indeed the inadequate positioning of the device initially or device migration thereafter may be a risk factor of IE. However the device was performed adequately in this patient with the device implanted 6-8 mm beneath the aortic annulus (Fig. 2). Moreover, there has been no case of device migration after successful device deployment6)7) and we also could not find definite evidence of device migration by echocardiography. Besides, the degree of paravalvular AR had not changed at follow-up, which is difficult to imagine in the case of significant prosthesis migration. Therefore, the possibility of IE caused by device migration may be dismissed in this case.
According to the literature, IE after TAVR seems to be very rare,8) but it is also a very serious complication,9) the majority of which requires surgical intervention.10)11)12)13) The diagnosis of IE after TAVR might be particularly difficult and often delayed, therefore precise and early diagnosis is required. According to the Duke criteria for diagnosis of IE, the major criteria are typical microorganisms consistent with IE and echocardiography findings positive for IE, such as vegetation, abscess or new partial dehiscence of prosthetic valve.14) Because of the limited experience with IE associated with TAVR, the following points remain to be discussed in the future. First, although Streptococcus anginosus, which is a subtype of Streptococcus viridans-the common pathogen for IE especially in native valve endocarditis-was identified in this patient, the common pathogen associated with IE in TAVR is yet to be investigated. Second, there are some structural differences between surgical valve and transcatheter valve. Like our case, echocardiographic findings related to IE could be obscured by the metallic struts encircling the valve leaflets9) and there are no sutures that connect the bioprosthesis and the aortic annulus. Therefore, valvular dehiscence may not be that common as in surgical valves and a more meticulous examination should be performed at multiple views during echocardiography for any evidence of vegetation or abscess pocket. Therefore we anticipate that echocardiography may play a more critical and important role in these types of setting.
This patient did not perform dental check-up before TAVR, and underwent a dental procedure without antibiotics. Currently, there are no guidelines for prophylaxis of IE after TAVR.12) Only a few case reports have suggested that the dental procedure and the lack of endocarditis prophylaxis may be a precipitating factor of IE in TAVR setting.11)12) We also agree with our limited experience that dental procedure without antibiotics prophylaxis may be a predisposing factor for IE after TAVR. Furthermore considering this possibility, proper antibiotics before the dental procedure should be considered, just as a prosthetic cardiac valve replaced by surgery in the current guidelines.15)
In conclusion, we report an interesting case of IE 1 month after successful TAVR. The location of the vegetation and subsequent mitral regurgitation suggests that the local mechanical irritation by the valve strut and even mild paravalvular AR might play a possible role for IE initiation after TAVR. Also, antibiotics prophylaxis should be considered strongly in patients who have undergone TAVR just like the patients who have had surgical valve replacement. Data, preferably international, on this important issue should be gathered and analyzed in the future.
Figures and Tables
Fig. 1
Transthoracic echocardiography images before and after transcatheter aortic valve replacement (TAVR). A and B: Apical 5-chamber views with color Doppler images. C and D: Parasternal short-axis views of the aortic valve. E and F: Continuous wave Doppler of aortic valve. A, C, and E: Before TAVR. B, D, and F: After TAVR.
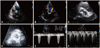
Fig. 2
Angiographic image just after transcatheter aortic valve replacement. The device was implanted 6-8 mm deep into the left ventricle, which demonstrates the adequacy of implantation per manufacturer's recommendation.

Fig. 3
A: Simple chest X-ray revealed bilateral pleural effusion. B: 12-lead electrocardiogram showed atrial fibrillation with rapid ventricular response and left bundle branch block.

Fig. 4
Transthoracic and transesophageal echocardiography (TEE) images of the aortic valve at the time of diagnosis of infective endocarditis. A: Parasternal long-axis images of the mitral valve. Significant mitral regurgitation can be seen just beneath the strut of the bioprosthetic aortic valve (green arrow). B: TEE images show that the vegetation (blue arrow) is located just at the ventricular side of the aortic valve bioprosthesis with subsequent perforation of the anterior mitral valve. There is also thickening of the aortomitral continuity (red arrow) suggestive of abscess due to endocarditis.

References
1. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011; 364:2187–2198.

2. Loh PH, Bundgaard H, S Ndergaard L. Infective endocarditis following transcatheter aortic valve replacement-: diagnostic and management challenges. Catheter Cardiovasc Interv. 2013; 81:623–627.

3. Raschpichler M, Seeburger J, Strasser RH, Misfeld M. Corevalve prosthesis causes anterior mitral leaflet perforation resulting in severe mitral regurgitation and subsequent endocarditis. Eur Heart J. 2014; 35:1587.

4. Abdel-Wahab M, Zahn R, Horack M, Gerckens U, Schuler G, Sievert H, Eggebrecht H, Senges J, Richardt G. German transcatheter aortic valve interventions registry investigators. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart. 2011; 97:899–906.

5. Puls M, Eiffert H, Hünlich M, Schöndube F, Hasenfuß G, Seipelt R, Schillinger W. Prosthetic valve endocarditis after transcatheter aortic valve implantation: the incidence in a single-centre cohort and reflections on clinical, echocardiographic and prognostic features. EuroIntervention. 2013; 8:1407–1418.

6. Linke A, Wenaweser P, Gerckens U, Tamburino C, Bosmans J, Bleiziffer S, Blackman D, Schäfer U, Müller R, Sievert H, Søndergaard L, Klugmann S, Hoffmann R, Tchétché D, Colombo A, Legrand VM, Bedogni F, Leprince P, Schuler G, Mazzitelli D, Eftychiou C, Frerker C, Boekstegers P, Windecker S, Mohr FW, Woitek F, Lange R, Bauernschmitt R, Brecker S. For the ADVANCE study Investigators. Treatment of aortic stenosis with a self-expanding transcatheter valve: the International Multi-centre ADVANCE Study. Eur Heart J. 2014; [Epub ahead of print].

7. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK. U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014; 370:1790–1798.

8. Carnero-Alcázar M, Maroto Castellanos LC, Carnicer JC, Rodríguez Hernández JE. Transapical aortic valve prosthetic endocarditis. Interact Cardiovasc Thorac Surg. 2010; 11:252–253.

9. Eisen A, Shapira Y, Sagie A, Kornowski R. Infective endocarditis in the transcatheter aortic valve replacement era: comprehensive review of a rare complication. Clin Cardiol. 2012; 35:E1–E5.

10. Head SJ, Dewey TM, Mack MJ. Fungal endocarditis after transfemoral aortic valve implantation. Catheter Cardiovasc Interv. 2011; 78:1017–1019.

11. Wong DR, Boone RH, Thompson CR, Allard MF, Altwegg L, Carere RG, Cheung A, Ye J, Lichtenstein SV, Ling H, Webb JG. Mitral valve injury late after transcatheter aortic valve implantation. J Thorac Cardiovasc Surg. 2009; 137:1547–1549.

12. Castiglioni A, Pozzoli A, Maisano F, Alfieri O. Endocarditis after transfemoral aortic valve implantation in a patient with Osler-Weber-Rendu syndrome. Interact Cardiovasc Thorac Surg. 2012; 15:553–554.

13. Comoglio C, Boffini M, El Qarra S, Sansone F, D'Amico M, Marra S, Rinaldi M. Aortic valve replacement and mitral valve repair as treatment of complications after percutaneous core valve implantation. J Thorac Cardiovasc Surg. 2009; 138:1025–1027.

14. Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Moreillon P, de Jesus Antunes M, Thilen U, Lekakis J, Lengyel M, Müller L, Naber CK, Nihoyannopoulos P, Moritz A, Zamorano JL. ESC Committee for Practice Guidelines. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009; 30:2369–2413.
15. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, Bolger A, Cabell CH, Takahashi M, Baltimore RS, Newburger JW, Strom BL, Tani LY, Gerber M, Bonow RO, Pallasch T, Shulman ST, Rowley AH, Burns JC, Ferrieri P, Gardner T, Goff D, Durack DT. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee. American Heart Association Council on Cardiovascular Disease in the Young. American Heart Association Council on Clinical Cardiology. American Heart Association Council on Cardiovascular Surgery and Anesthesia. Quality of Care and Outcomes Research Interdisciplinary Working Group. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007; 116:1736–1754.
Supplementary movie legends
Movie 1-4. Transthoracic echocardiography images before (Movie 1 and 3) and after (Movie 2 and 4) transcatheter aortic valve replacement (TAVR). Apical 5-chamber views with color Doppler images (Movie 1 and 2). Parasternal short-axis views of the aortic valve (Movie 3 and 4). Before TAVR (Movie 1 and 3). After TAVR (Movie 2 and 4).
Movie 5. Parasternal long-axis images of the mitral valve showing mitral regurgitation.
Movie 6. Transesophageal echocardiography images showing the vegetation located just at the ventricular side of the aortic valve bioprosthesis with subsequent perforation of the anterior mitral valve.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download