Abstract
Pulmonic valve infective endocarditis in isolation is a rare clinical entity. The formation of an abscess in the right ventricular outflow tract as a consequence of vegetations affecting the pulmonic valve in a structurally normal heart is extremely rare and has not been reported. We report a case of isolated pulmonic valve endocarditis complicated by a regional abscess formed within the right ventricular outflow tract caused by Streptococcus Constellatus (S. Constellatus), a member of the Streptococcus Milleri group in a young male whose risk factor was alcohol abuse and he was treated medically, a comprehensive literature review on the subject is also reported. Our case is the first reported in literature with infective endocarditis caused by S. Constellatus affecting the pulmonic valve, and the first with pulmonic valve endocarditis and perivalvular abscess formation in a structurally normal heart.
Infective endocarditis affecting the right heart accounts for 5-6% of total cases of infective endocarditis with the majority affecting the tricuspid valve either in isolation or involving the pulmonic valve as a sequel. Endocarditis involving the pulmonic valve in isolation has been reported as 1.5-2% of total cases of infective endocarditis.1) Since the 1960s and up to 2012, 82 cases were reported in literature. The majority of cases were in the pediatric population with congenital heart diseases.2)3) Historically, risk factors of pulmonic valve endocarditis have been described as intravenous drug abuse, alcoholism, sepsis, central venous catheters, intra-cardiac devices and congenital heart diseases with prior repairs. In 28% of cases, no definite risk factors have been identified.4) Transthoracic echocardiography (TTE) and transeosophageal echocardiography (TEE) are the cornerstones for establishing the diagnosis. Vegetations are the hallmark lesions of infective endocarditis. Abscesses are the second major echocardiographic criterion for endocarditis and they are more frequently observed in aortic valve infective endocarditis and usually involve the mitral-aortic intervalvular fibrosa.5) The finding of an abscess in the right ventricular outflow tract is also rare and commonly involves pulmonic grafts and prosthesis in the right ventricular outflow tract position. Here we are describing a 37-year-old man who presented with an isolated pulmonic valve endocarditis complicated by perivalvular abscess formed in the right ventricular outflow tract. Comprehensive review of literature is also reported.
A 37-year-old man with past medical history remarkable for type tow diabetes on insulin therapy, heavy smoking, alcohol dependence with multiple admissions for severe alcohol withdrawal, chronic pancreatitis related to alcohol and depression with previous admissions for suicidal attempts. He denied any recent travel, animal exposure or intravenous drug use. His tuberculosis and human immunodeficiency virus screen were unremarkable. He was admitted to a peripheral hospital with symptoms and signs of pneumonia, extensive workup had revealed right upper lobar pneumonia with positive blood cultures for Actinomyces and gram negative bacilli, he was initially on ceftriaxone and later switched to penicillin and metronidazole. Under this regimen, his general condition worsened over the subsequent few days. A week later, he was transferred to the intensive care unit (ICU) of our tertiary care hospital. Upon arrival, his temperature was 39℃, respiratory rate 55/min, heart rate was 130 bpm, blood pressure 120/75 mmHg and his oxygen saturation was 90% on 15 L of O2 non-rebreather mask. During his ICU stay, he developed acute respiratory distress syndrome secondary to diffuse bilateral pneumonia and Type I hypoxic respiratory failure, thus he was intubated and mechanically ventilated on FiO2 100%. His antibiotics were then changed to Piperacillin/Tazobactam and Vancomycin, awaiting blood culture results. Biochemically, he had an elevated international normalized ratio but a normal creatinine. His liver enzymes were somewhat remarkable for an elevated alkaline phosphatase at 397 with a gamma-glutamyl transferase of 200. Computed tomography (CT) scan of his chest revealed; diffuse bilateral infiltrate. During his workup within the context of pneumonia, ultimately he did grow bacteria that were consistent with Streptococcus Milleri (S. Milleri) group and ultimately speciated as Streptococcus Constellatus (S. Constellatus) sensitive to Penicillin. Due to persistent bacteremia and sepsis, a TTE was requested to rule out endocarditis. The TTE revealed an echo-bright mass (1.5 × 1.5 cm) attached to the pulmonic valve consistent with vegetation, there were neither stenosis nor valvular insufficiency of the pulmonic valve, mild tricuspid regurgitation and an elevated right ventricular systolic pressure to 50-60 mmHg (Fig. 1). A stat TEE revealed multiple mobile echogenic targets arising from the pulmonic valve consistent with vegetations (Fig. 2). The diagnosis of infective endocarditis was established, and based on blood culture results, penicillin G was started. Over the next five days, the patient remained febrile. A follow-up TEE showed vegetations on the pulmonic valve that has increased in size with an echogenic appearance within the posterior wall of the right ventricular outflow tract consistent with an abscess without fistulous communication (Fig. 3), the pulmonic valve function was intact and all other valves (Supplementary movie 1) were apparently normal in structure and function. CT-brain, chest, abdomen, and pelvis-revealed multiple relatively sub-acute but recent left temporal and frontal infarctions. There were also multiple ring enhancing lesions scattered throughout the cerebrum and there was no evidence of additional abscesses. The day after that, his general condition improved, fever subsided, lung condition improved requiring less FiO2 and he was successfully extubated after three days. At this point of time, multidisciplinary teams including infectious diseases, cardiology and cardiac surgery were involved in his care. The infectious diseases service did comment on the propensity of S. Milleri with persistent bacteremia to potentially seed to alternative places in the body and this was unlikely related to pulmonic valve endocarditis. This further reinforced the opinion of cardiac surgeons that he is not an operative candidate. Serial TEEs were performed and did not show extension of the abscess neither to the septum nor to the aortic valve. Ultimately, he was treated conservatively with penicillin G for total of 6 weeks. He developed neurological deficits in the form of partial expressive aphasia, agaraphia requiring prolonged speech therapy, physiotherapy and rehabilitation. One month later, a follow-up TEE showed a friable serpiginous mass in the pulmonic valve that had shrunken in size likely representing healed vegetation and no abscess was identified (Fig. 4).
Isolated pulmonic valve endocarditis is a very rare clinical entity. Theoretically, this might be related to the low oxygen content of the venous blood, the low pressure in the right heart and to the differences in the endothelial covering and the vascularization of the right heart, thus the higher prevalence is among the population of congenital heart diseases with predominant left to right shunts.2) In our patient, alcoholism and probably diabetes were the major predisposing risk factors due to their proven negative impact on the immune system at multiple cellular levels.6) The patient denied any history of intra-venous drug abuse. The primary source of infection was likely the community acquired pneumonia which forms the path via which the bacteria harvest the blood and find its way to affect the heart valves.
Staphylococcus Aureus is by the far the commonest pathogen reported in cases of pulmonic valve endocarditis, followed by (group B Streptococci), Lactobacillus species. Pseudallescheria Boydii, Candida Albicans, and Actinobacillus actinomycetemcomitans were isolated. Few cases due to Enterococci were also reported.7) Pasteurella Multocida cases have been reported with associated valvular destruction.8) S. Milleri is an unofficial name applied to a group of basically similar viridians Streptococci species showing various hemolytic, serological, and physiological characteristics. S. Milleri strains display a range of characteristics that may be important pathogenic determinants in the formation of both abscesses and endocarditis. Members include Streptococcus Anginosus, S. Constellatus; S. Constellatus subsp. Constellatus and S. Constellatus subsp. Pharyngis and Streptococcus Intermedius. S. Constellatus was the only species able to produce thrombin-like activity, the Lancefield group C strains were the only strains capable of aggregating platelets9) and infective endocarditis causation is extremely rare. Few cases are reported in the literature with infective endocarditis caused by S. Constellatus affecting the mitral, aortic, and prosthetic valves. Ejima et al.10) described prosthetic valve endocarditis caused by S. Constellatus infection complicated by multiple organ failure and systemic embolism. They considered that surgical treatment was difficult, continued antibiotic therapy, and at follow-up the patient developed paravalvular abscesses around the aortic valve. Our case is the first reported in the literature with infective endocarditis affecting the pulmonic valve due to S. Constellatus. S. Constellatus are generally sensitive to penicillin, although resistant strains are evolving; our patient initially was covered by vancomycin and piperacillin/tazobactam in the ICU in the context of his pneumonia and sepsis which was later changed to penicillin G for 6 weeks following the establishment of infective endocarditis diagnosis for which he showed good clinical response.
The detection of vegetations by 2-dimensional TTE with a resolution size of 3-4 mm has a sensitivity of 62-79% and a specificity of 91-100% and this depends on the quality of images available. TEE has a higher sensitivity (87-100%) for detection of smaller size vegetations 1-2 mm in diameter and specificity similar to TTE.11)12) Therefore, in our case the initial size measured was 15 × 15 mm (Fig. 1) which was easily detected by TTE while on the follow-up TEE, the abscess was clearly visualized in the posterior wall of the right ventricular outflow tract described as a thickened non-homogeneous perivalvular area with an echo-dense appearance. The location of the abscess in the right ventricular outflow tract is somehow unusual, give the fact that it is in reverse direction of blood flow, and theoretically it should form in the pulmonary artery. The sensitivity of TTE for the diagnosis of abscesses is about 50%, compared to 90% for TEE. Specificity higher than 90% has been reported, for both TTE and for TEE. Therefore, TEE needs to be performed in all cases of infective endocarditis and as soon as an abscess is suspected.13) Larger vegetations are potentially embolic, although the patient had multiple strokes, still the mechanism is unclear given the fact that left sided valves were not affected, there was no intra-cardiac shunt identified, and this was further confirmed by a negative saline bubble study. However, other atherosclerotic risk factors were present, i.e., diabetes and smoking history which might be incriminated. The patient had a good clinical response to medical therapy. However, the option of surgical intervention was thoroughly discussed in multi-disciplinary meetings involving the patient and his family members and the final decision was to treat him medically based on patient's preference.
Isolated pulmonic valve endocarditis presents a great challenge to cardiac surgeons due to poor postoperative compliance and high relapse rate.14) Surgical techniques, i.e., 'prosthetic' valve implantation, have been reported with satisfactory results.15) Considering that prosthetic valve replacement theoretically exposes the patients to valve-related complications and to some risk of recurrent endocarditis, 'non-prosthetic' surgical techniques with pulmonary valve repair using autologous pericardial patch were applied with satisfactory outcomes.14) In our case, the size of vegetation and the abscess in the right ventricular outflow tract might have been a reasonable indication to go for surgery especially the persistence of vegetation following the completion of antibiotics regimen. However, the functionality of the pulmonic valve has been well maintained throughout his illness and from clinical point of view, the patient has had a satisfactory recovery.
In Conclusion, pulmonic valve endocarditis is a very rare clinical entity. The diagnosis of infective endocarditis affecting the pulmonic valve is a persistent challenge. There are no uniform guidelines and/or consensus agreement on the treatment of pulmonic valve endocarditis. Both medical and surgical approaches have shown favorable results in the current era and we suggest that, clinical decisions should be individualized based on clinical profile. The pulmonic valve is very thin and pliable, not perfectly visualized by echocardiogram most of the times and sometimes "ignored". We advise that, physicians should always interrogate the pulmonic valve thoroughly when interpreting echocardiogram to rule out endocarditis.
Figures and Tables
Fig. 1
Transthoracic echocardiogram. Left parasternal short axis view at the level of the aortic valve showing (arrow) large sized vegetation attached to the pulmonic valve.
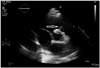
Fig. 2
Transoesophageal echocardiogram. Mid-oesophageal short axis view at the level of the aortic valve showing (arrow) large sized vegetation attached to the right ventricular outflow tract side of the pulmonic valve. A: tricuspid valve appears normal in structure.
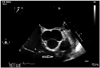
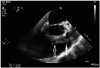
Fig. 3
Transoesophageal echocardiogram. Mid-oesophageal right ventricular outflow view showing large sized vegetations attached to the right ventricular outflow tract (RVOT) side of the pulmonic valve (solid arrow). An abscess within the posterior wall of the RVOT, note the echo free spaces within (open arrow).
References
1. Cassling RS, Rogler WC, McManus BM. Isolated pulmonic valve infective endocarditis: a diagnostically elusive entity. Am Heart J. 1985; 109(3 Pt 1):558–567.

2. Ramadan FB, Beanlands DS, Burwash IG. Isolated pulmonic valve endocarditis in healthy hearts: a case report and review of the literature. Can J Cardiol. 2000; 16:1282–1288.
3. Schroeder RA. Pulmonic valve endocarditis in a normal heart. J Am Soc Echocardiogr. 2005; 18:197–198.

4. Cremieux AC, Witchitz S, Malergue MC, Wolff M, Vittecocq D, Vilde JL, Frottier J, Valere PE, Gibert C, Saimot AG. Clinical and echocardiographic observations in pulmonary valve endocarditis. Am J Cardiol. 1985; 56:610–613.

5. Habib G, Badano L, Tribouilloy C, Vilacosta I, Zamorano JL, Galderisi M, Voigt JU, Sicari R, Cosyns B, Fox K, Aakhus S. European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. 2010; 11:202–219.

6. Moreira D, Correia E, Rodrigues B, Santos L, Capelo J, Abreu L, Nunes L, Oliveira-Santos J. Isolated pulmonary valve endocarditis in a normal heart. Rev Port Cardiol. 2012; 31:615–617.

7. Hamza N, Ortiz J, Bonomo RA. Isolated pulmonic valve infective endocarditis: a persistent challenge. Infection. 2004; 32:170–175.

8. Graf S, Binder T, Heger M, Apfalter P, Simon N, Winkler S. Isolated endocarditis of the pulmonary valve caused by Pasteurella multocida. Infection. 2007; 35:43–45.

9. Willcox MD. Potential pathogenic properties of members of the "Streptococcus milleri" group in relation to the production of endocarditis and abscesses. J Med Microbiol. 1995; 43:405–410.

10. Ejima K, Ishizuka N, Tanaka H, Tanimoto K, Shoda M, Kasanuki H. [Prosthetic valve endocarditis caused by Streptococcus constellatus infection complicated with perivalvular abscess: serial observation by transesophageal echocardiography: a case report]. J Cardiol. 2003; 42:129–133.
11. Pedersen WR, Walker M, Olson JD, Gobel F, Lange HW, Daniel JA, Rogers J, Longe T, Kane M, Mooney MR, et al. Value of transesophageal echocardiography as an adjunct to transthoracic echocardiography in evaluation of native and prosthetic valve endocarditis. Chest. 1991; 100:351–356.

12. Jacob S, Tong AT. Role of echocardiography in the diagnosis and management of infective endocarditis. Curr Opin Cardiol. 2002; 17:478–485.

13. Daniel WG, Mügge A, Martin RP, Lindert O, Hausmann D, Nonnast-Daniel B, Laas J, Lichtlen PR. Improvement in the diagnosis of abscesses associated with endocarditis by transesophageal echocardiography. N Engl J Med. 1991; 324:795–800.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download