Abstract
A 51-year-old highly fit man presented for dyspnea with strenuous aerobic exercise. The patient was asymptomatic and all tests were normal at rest. With increasing exercise intensity, he suddenly complained of dyspnea and showed a severe exercise-induced hypoxemia with an excessive alveolar-arterial oxygen tension difference. In agitated saline contrast echocardiography at peak exercise, a large amount of left to right shunt was identified after > 5 cardiac cycles, which suggests the presence of exercise-induced intrapulmonary arteriovenous shunt in this patient.
The decline in PaO2 and SaO2 during exercise has been termed as exercised-induced arterial hypoxemia (EIAH) and has been classified into mild (arterial oxygen saturation, SaO2 = 93-95%), moderate (SaO2 = 88-93%), and severe (SaO2 < 88%).1)
EIAH has been well documented in highly fit male endurance athletes during heavy and even submaximal exercise and have been reported to occur in approximately 50% of highly trained runners.2) However, the determining factor which is responsible for the development of EIAH in those patients is unclear. We report a highly fit male complaining of dyspnea during strenuous exercise who underwent cardiopulmonary treadmill exercise test and agitated saline contrast echocardiography at peak exercise.
A 51-year-old man presented to the outpatient department because of dyspnea with strenuous aerobic exercise. As an amateur rollerblader, he has rollerbladed twice a week for the past 5 years. About 9 months ago, dyspnea occurred abruptly during rollerblading at full speed for over ten minutes. At that time, he felt like collapsing but his symptom improved completely within 3 minutes after stopping the exercise. He had no history of known airway or cardiovascular disease.
At rest, he was asymptomatic and physical examination revealed clear lung sound and normal heart sounds without murmurs. Electrocardiography, chest radiography, and blood tests were normal. Oxygen saturation was 99 at room air and pulmonary function test was also normal at rest. A transthoracic echocardiogram revealed normal cardiac structure and function. After a baseline echocardiogram, agitated saline contrast echocardiography was performed to evaluate intra- or extracardiac shunt as a cause of the dyspnea. Only a few air bubbles appeared in the left heart after 9 cardiac cycles from the onset of right heart opacification (Fig. 1). To assess for the presence of intrapulmonary (IP) shunt, such as pulmonary arteriovenous malformation (AVM), computed tomography of the chest (Fig. 2A) and pulmonary angiography (Fig. 2B) were performed and they showed no significant abnormality. Since the patient complained of dyspnea during strenuous exercise, a cardiopulmonary treadmill exercise test (Quark b2, COSMED, Rome, Italy) with arterial blood gas analysis (ABGA) was performed. While increasing the exercise intensity, the patient suddenly complained of dyspnea at 205 watts of exercise and the values obtained from cardiopulmonary exercise test and ABGA at that time were as follows (Table 1). We confirmed the presence of severe exercise-induced arterial hypoxemia and an excessive alveolar-arterial oxygen tension difference (A-aDO2) widening in this patient. Bilateral breathing sounds were normal in auscultation during the dyspneic episode.
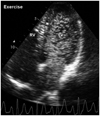
When an exercise echocardiography was done within one minute after stopping the exercise, there was no evidence of exercise-induced deterioration of cardiac function. However, the agitated saline contrast echocardiography revealed that large amount of microbubbles appeared in the left atrium and leaded to complete left heart opacification after > 5 cardiac cycles from the onset of right heart opacification (Fig. 3), suggesting the presence of exercise-induced IP shunts. The patient's dyspnea and hypoxemia in pulse oxymetry were completely normalized within one minute of rest. He has been limiting strenuous exercise for one year and remains free of symptoms.
This patient showed a severe EIAH with a widening of A-aDO2 during strenuous exercise. The severity of EIAH is significantly correlated with gas-exchange efficiency as quantified by the A-aDO2. Potential mechanisms of EIAH with a widening of A-aDO2 include ventilation-perfusion mismatching, diffusion limitation, and right-to-left shunting.1)
Although prior studies, using the multiple inert gas elimination techniques and/or inspired 100% oxygen have failed to detect significant right-to-left physiological IP shunt during exercise,3)4) there are substantial studies documenting the presence of anatomical arteriovenous anastomoses in the isolated lung and the recruitment of IP shunts during exercise using the agitated saline contrast echocardiography technique.5)6) The recruitment of IP shunt might be a response to increasing pulmonary arterial pressure and/or cardiac output during dynamic exercise to reduce the potential damaging effects of high perfusion pressure during exercise.6) Also, recent studies have reported that oxygen tension might play a crucial role in the regulation of these shunt vessels.7) However, determinants regulating these shunt vessels during exercise in some highly fit subjects and their contribution to the components of gas exchange has not been fully elucidated.
The present case indicates that the existence of exercise-induced IP shunt has a close connection with dyspnea by impairing gas exchange with widening of the A-aDO2. Additionally, considering the lung's role as a biologic filter and the increased risk of stroke in patients with pulmonary AVM, the recognition of exercise-induced IP shunt among patients complaining of exertional dyspnea is crucial because this condition might serve as another potential source of embolic stroke.
Figures and Tables
Fig. 1
The agitated saline contrast echocardiography at rest showed only a few microbubbles appearing in the left heart after 9 cardiac cycles from the onset of right heart opacification.
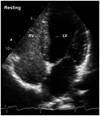
Fig. 2
Computed tomography of the chest (A) and pulmonary angiography (B) to assess the presence of intrapulmonary shunt, such as pulmonary arteriovenous malformation showed no significant abnormality.
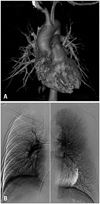
Fig. 3
Agitated saline contrast echocardiography done at the time the patient complained of dyspnea revealed large amount of microbubbles appearing in the left atrium and lead to complete left heart opacification after > 5 cardiac cycles from the onset of right heart opacification.
References
1. Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol (1985). 1999; 87:1997–2006.

2. Powers SK, Dodd S, Lawler J, Landry G, Kirtley M, McKnight T, Grinton S. Incidence of exercise induced hypoxemia in elite endurance athletes at sea level. Eur J Appl Physiol Occup Physiol. 1988; 58:298–302.

3. Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol (1985). 1986; 61:260–270.

4. Vogiatzis I, Zakynthinos S, Boushel R, Athanasopoulos D, Guenette JA, Wagner H, Roussos C, Wagner PD. The contribution of intrapulmonary shunts to the alveolar-to-arterial oxygen difference during exercise is very small. J Physiol. 2008; 586:2381–2391.

5. Lovering AT, Stickland MK, Kelso AJ, Eldridge MW. Direct demonstration of 25- and 50-microm arteriovenous pathways in healthy human and baboon lungs. Am J Physiol Heart Circ Physiol. 2007; 292:H1777–H1781.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download