Abstract
Stress cardiomyopathy (SCMP) is characterized by a transient left ventricular dysfunction associated with apical ballooning and compensatory hyperkinesias of the basal segments after emotional or physical stress, but inverted or mid-ventricular variants of SCMP have also been described. Although catecholamine excess has been suggested as a possible pathophysiologic mechanism of SCMP, the etiology of SCMP is still unknown. Here, we report a case of inverted type of SCMP with clinical presentation mimicking acute coronary syndromes. The cause or precipitating stressor was unclear initially, but pheochromocytoma has been demonstrated as a cause of SCMP during clinical follow-up at out-patient clinic in the present case. Catecholamine-producing tumors should be included in the evaluation or management of SCMP, even though initial clinical manifestations are not suggestive for pheochromocytoma.
Stress cardiomyopathy (SCMP), also known as takotsubo cardiomyopathy or apical ballooning syndrome, is now a well established disease entity and characterized by rapid and severe reversible cardiac dysfunction without significant coronary arterial stenosis.1,2,3) Emotional or physical stressors have been described as triggering factors of this syndrome, but these precipitating stressors are not identified in some cases. Recently, catecholamine excess has been implicated as a possible pathophysiologic mechanism of SCMP, but the association between catecholamine excess and SCMP is unclear.4)
Pheochromocytoma is a rare neuroendocrine tumor of catecholamine producing chromaffin cells.5) Secondary hypertension with features of paroxysm and cold sweating is common clinical presentation of pheochromocytoma, but various patterns of cardiomyopathy including SCMP have been described.
Here, we report a case of inverted type of SCMP with clinical presentation mimicking acute coronary syndromes. The cause or precipitating stressor was unclear initially, but pheochromocytoma has been demonstrated as a cause of SCMP during clinical follow-up at out-patient clinic in the present case.
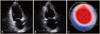
A 45-year-old male presented with substernal squeezing chest pain which developed 10 hours ago. He had been treated for hypertension with anti-hypertensive agents intermittently for last 4 years at private clinic, and the patient stopped antihypertensive medication arbitrarily for recent 2 months. On admission, blood pressure (BP) was 180/100 mmHg, and heart rate was 80 beats/min. Physical examinations were non-specific, and chest X-ray findings was also non-specific. Electrocardiography showed upright tall T-wave and prolonged QT interval (corrected QT interval: 467 msec) without ST segment elevations, and the electrocardiographic abnormalities were normalized before discharge. Cardiac biomarkers were elevated; 2.56 ng/mL for cardiac troponin I, 2944 pg/mL for N-terminal pro-B-type natriuretic peptide. Other laboratory findings were unremarkable. With the clinical diagnosis of acute non-ST elevation myocardial infarction, coronary angiography was performed, but there were no stenosis on both coronary arteries. Echocardiography was performed and revealed dyskinesia of the left ventricular (LV) basal segments and compensatory hyperkinesia of mid to apical LV segments (Fig. 1 A and B). Strain echocardiography revealed mostly positive longitudinal strain values of the basal segments and normal strain values of the mid to apical segments (Fig. 1 C, Supplementary movie 1). Conservative medical managements including angiotensin converting enzyme inhibitor (ACEI) and beta-blocker (BB) for inverted type of SCMP and hypertension were done. Because BP of the patient became hypotensive after 1 days of medication, ACEI and BB were stopped. Follow-up echocardiography after 5 days revealed normalized LV wall motions and systolic function (Supplementary movie 2). BP was maintained below 120/80 mmHg during hospitalization. The patient was improved and discharged without anti-hypertensive medications.
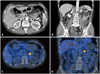
During first 2 months of out-patient clinic follow-up after discharge, the patient was free of symptoms and office BP was maintained below 120/80 mmHg. Thereafter, however, the patient complained of intermittent palpitation and headache. The patient revisited emergency room owing to headache and palpitation, and BP at emergency room was 220/130 mmHg. At this time, past medical histories of the patient reviewed again and revealed that the patient visited emergency room 4 times because of paroxysmal marked elevation of BP, palpitation, and sweating for last 4 years. Chest X-ray finding was non-specific, and electrocardiography showed normal sinus rhythm without ST-T wave abnormalities or QT prolongation. Echocardiography revealed good LV systolic function without regional wall motion abnormalities. Serum hormonal studies including norepinephrine, epinephrine, renin activity, aldosterone showed no abnormal elevations. Urine hormonal studies collected from 24 hours revealed marked elevations of catecholamines and their metabolites; norepinephrine: 1259.6 ug/day (15-80 ug/day), epinephrine: 647.9 ug/day (0-20 ug/day), metanephrine: 10.5 mg/day (0-0.8 mg/day), vanillylmandelic acid: 14.8 mg/day (0-8 mg/day), but the level of 24 hour urinary free cortisol was normal. Abdominal computed tomography revealed about 2.5 cm sized homogeneously enhancing mass on left adrenal gland (Fig. 2A and B), and I-123 metaiodobenzylguanidine (MIBG) scan revealed focal MIBG uptake on left adrenal gland consistent with pheochromocytoma (Fig. 2C and D).
Laparoscopic adrenalectomy was done successfully. The symptoms and signs of pheochromocytoma were not recurred, and BP of the patient was maintained within normal range without antihypertensive agents for 12 months of clinical follow-up.
The present case gives several clinically important educational messages in the evaluation or management of SCMP. Firstly, pheochromocytoma should be included and thus serum and urinary catecholamines should be measured in the evaluation of SCMP, even though the symptoms or signs were not suggestive of pheochromocytoma. Secondly, SCMP associated with pheochromocytoma may not be a typical pattern of SCMP as shown in the present case.
Although SCMP has been reported from the early 1990s, the cause or pathophysiologic mechanism of SCMP is still unclear.1,2,3) Various emotional or physical stressors have been described as predisposing conditions of SCMP, but these precipitating stressors, as in the present case, are not identified in some cases. Recently, catecholamine excess has been implicated as a possible pathophysiologic mechanism of SCMP, but the association between catecholamine excess and SCMP is also unclear.4) Because catecholamine excess in the setting of pheochromocytoma might be a possible explanation of SCMP in the present case, it is assumed that catecholamine plays a role in the pathogenesis of SCMP, even though not applicable to all cases of SCMP. Therefore, the measurements of catecholamines and their metabolites in patients with SCMP would be helpful and should be considered, even in patients without typical symptoms or signs of pheochromocytoma.
Pheochromocytoma is a rare neuroendocrine tumor of catecholamine producing cells located in adrenal medulla.5) Hypertension, either continuous or episodic, is the most common feature of pheochromocytoma, but variable patterns of cardiomyopathy including SCMP has been described in the literature.6,7,8) Typical pattern of SCMP is characterized by a transient cardiac dysfunction associated with apical dyskinesia or akinesia and compensatory basal hyperkinesias, so called takotsubo contractile pattern, after an emotional or physical stress. However, the distribution of segmental wall motion abnormalities other than apical segments such as mid-ventricular or inverted variant of SCMP has also been demonstrated.1)2) In the literature, as shown in the present case, an inverse takotsubo contractile pattern has been more commonly described than takotsubo contractile pattern in patients with pheochromocytoma-induced SCMP.9)10) Recently, transient mid-ventricular ballooning cardiomyopathy associated with bladder pheochromocytoma was also described in the literature.11) Considering the previous reports, it is suggested that the evaluation of pheochromocytoma should be included in patients with SCMP, especially in inverted variants of SCMP. For this reason, the absence of pheochromocytoma has been included in the Mayo clinic criteria for the diagnosis of transient LV apical ballooning syndrome.12)
The authors did not consider pheochromocytoma as a possible cause of SCMP initially, because the symptoms or signs suggestive of pheochromocytoma were not seen at initial clinical presentation. Because pheochromocytoma has a characteristic of paroxysm in clinical presentation, high index of suspicion and systematic complete history taking should be done in patients with SCMP. Routine measurements of catecholamines and their metabolites may minimize the mistake overlooking pheochromocytoma as a hidden cause of SCMP.
In conclusion, we report a case of inverted type of SCMP with clinical presentation mimicking acute coronary syndromes. The cause or precipitating stressor was unclear initially, but pheochromocytoma has been demonstrated as a cause of SCMP during clinical follow-up at out-patient clinic in the present case. Pheochromocytoma should be included and thus serum and urinary catecholamines should be measured in the evaluation of SCMP, even though the symptoms or signs were not suggestive of pheochromocytoma.
Figures and Tables
Fig. 1
Echocardiography revealed akinesia or dyskinesia of the basal segments of the left ventricle on apical 2 chamber view (A: diastole, B: systole). Bull's eye mapping of two-dimensional speckle tracking strain imaging showed mostly positive longitudinal strain values of the basal segments and normal or increased strain values of the mid to apical segments (C).
References
1. Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, Honda T, Hase M, Kai R, Morii I. Angina Pectoris-Myocardial Infarction Investigations in Japan. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001; 38:11–18.

3. Hwang SH, Kim KH, Yoon HJ, Hong YJ, Kim JH, Ahn YK, Jeong MH, Cho JG, Park JC, Kang JC. Stress cardiomyopathy complicated by left ventricular thrombi and cerebral infarctions in a patient with essential thrombocythemia. J Cardiovasc Ultrasound. 2011; 19:87–90.

4. Abraham J, Mudd JO, Kapur NK, Klein K, Champion HC, Wittstein IS. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol. 2009; 53:1320–1325.

5. Renard J, Clerici T, Licker M, Triponez F. Pheochromocytoma and abdominal paraganglioma. J Visc Surg. 2011; 148:e409–e416.

6. Kassim TA, Clarke DD, Mai VQ, Clyde PW, Mohamed Shakir KM. Catecholamine-induced cardiomyopathy. Endocr Pract. 2008; 14:1137–1149.

7. Agarwal G, Sadacharan D, Kapoor A, Batra A, Dabadghao P, Chand G, Mishra A, Agarwal A, Verma AK, Mishra SK. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery. 2011; 150:1202–1211.

8. Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens. 2011; 29:2049–2060.

9. Rossi AP, Bing-You RG, Thomas LR. Recurrent takotsubo cardiomyopathy associated with pheochromocytoma. Endocr Pract. 2009; 15:560–562.

10. Kim S, Yu A, Filippone LA, Kolansky DM, Raina A. Inverted-Takotsubo pattern cardiomyopathy secondary to pheochromocytoma: a clinical case and literature review. Clin Cardiol. 2010; 33:200–205.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download