Abstract
Background
Abnormal interventricular septal motion (ASM) is frequently observed after open heart surgery (OHS). The aim of this study was to investigate the incidence and temporal change of ASM, and its underlying mechanism in patients who underwent OHS using transthoracic echocardiography (TTE).
Methods
In total, 165 patients [60 ± 13 years, 92 (56%) men] who underwent coronary bypass surgery or heart valve surgery were consecutively enrolled in a prospective manner. TTE was performed preoperatively, at 3--6-month postoperatively, and at the 1-year follow-up visit. Routine TTE images and strain analysis were performed using velocity vector imaging.
Results
ASM was documented in 121 of 165 patients (73%) immediately after surgery: 26 patients (17%) presented concomitant expiratory diastolic flow reversal of the hepatic vein, 11 (7%) had inferior vena cava plethora, and 11 (7%) had both. Only 2 patients (1%) showed clinically discernible constriction. ASM persisted 3--6 months after surgery in 38 patients (25%), but only in 23 (15%) after 1 year. There was no difference in preoperative and postoperative peak systolic strain of all segments of the left ventricle (LV) between groups with or without ASM. However, systolic radial velocity (VRad) of the mid anterior-septum and anterior wall of the LV significantly decreased in patients with ASM.
Conclusion
Although ASM was common (74%) immediately after OHS, it disappeared over time without causing clinically detectable constriction. Furthermore, we consider that ASM might not be caused by myocardial ischemia, but by the decreased systolic VRad of the interventricular septum after pericardium incision.
Abnormal interventricular septal motion (ASM) is the phenotype of exaggerated ventricular coupling, which is observed during pericardial constriction.1) Currently, the most common cause of pericardial constriction is previous cardiac surgery, followed by pericarditis, pericardial effusion, and radiotherapy.2)3) When the frequent occurrence of ASM after coronary bypass surgery (CBS) or heart valve surgery (HVS)4)5) is considered, relatively few studies have reported the incidence or causal mechanisms of ASM after cardiac surgery. Moreover, the results of these studies are not consistent and clinical implications. Therefore, the objective of this study was to investigate the incidence of ASM and its temporal evolution as well as the underlying mechanism determining its occurrence using transthoracic echocardiography (TTE).
Between June 2011 to May 2012, 203 patients underwent open heart surgery (OHS) (CBS or HVS) at Severance Cardiovascular Hospital. We excluded patients who had additional possible etiology for ASM, such as preexisting regional wall motion abnormality, left ventricular (LV) dysfunction, hypertrophic cardiomyopathy,6) previous pericardiectomy,7) postoperative severe tricuspid regurgitation,8) and conduction system-based abnormalities (permanent implanted pacemaker,9) left bundle branch block,10) or ventricular pre-excitation11)). The selection yielded a study group of 165 patients [60 ± 13 years, 92 men (56%)]. Patients were sorted into 2 groups according to presence of immediate postoperative ASM (ASM+) or its absence (ASM-). The term ASM is defined as the movement of the interventricular septum toward the right ventricle in systole with normal thickening (Fig. 1), which shows septal bouncing motion during systole.4)5) Clinical and echocardiographic parameters were analyzed.
Routine TTE images and strain analysis using velocity vector imaging (VVI) were obtained with a Acuson SC2000™ System and a Syngo® Sie VVI (Siemens Medical Solutions USA, Inc., Mountain View, CA, USA), respectively. Routine TTE and VVI analyses were performed before and immediately after OHS. Additional transesophageal echocardiography were performed at the 3--6- and 12-month follow-up visits to determine whether there was a serial change of ASM. The presence of ASM was measured with M-mode echocardiography and short axis view. Peak systolic circumferential strain (CS) and peak systolic radial velocity (VRad, cm/s) were used for VVI analysis.
Continuous variables were analyzed using Student's t-test, and dichotomous variables were analyzed using the chi square test. Data showed as mean ± standard deviations or number (%), and all variables that had a p value of 0.05 or less were considered statistically significant. We used SPSS for Macintosh, version 10.0.7a, (SPSS Inc., Chicago, IL, USA).
Among all the enrolled patients (n = 165) who underwent immediate post-operative echocardiography, 121 patients (73%) presented ASM immediately after OHS. Concomitant expiratory diastolic flow reversal of hepatic vein was found in 26 patients (17%), plethora of inferior vena cava in 11 (7%), and both in 11 (7%). However, clinically significant pericardial constriction, related to the subsequent use of diuretics and corticosteroids, was found only in 2 patients (1%). After 3--6 months of index post-operative echocardiography, 50 patients (30%, 50/165) did not perform echocardiography. ASM persisted in only 38 patients (33%, 38/115), and other concomitant findings had almost completely disappeared. One year later, ASM persisted in only 28 patients among patients who had follow-up echocardiography (25%, 28/109) (Fig. 2).
There were 121 patients who had ASM, resulting in an overall incidence of 73%. There were no statistically significant differences between the 2 groups regarding any of the baseline characteristics (Table 1).
Neither global nor regional CSs presented changes in patients in the ASM+ or in the ASM-groups, but systolic VRad of the antero-septum and anterior wall significantly decreased after surgery in patients in the ASM+ group (ΔVRad of the antero-septum: 0.6 ± 1.9 vs. 0.1 ± 1.2; p = 0.035 and anterior wall: 1.1 ± 1.9 vs. 0.1 ± 1.2; p = 0.002) (Table 4, Fig. 3).
ASM can be associated with many other conditions such as constrictive pericarditis,1) right ventricular overload,8) right ventricular pacing,9) left bundle branch block,10) septal ischemia or infarction, and congenital absence of the pericardium. Although these entities have different characteristics, their initial appearance by echocardiography may be similar. There are only few suggestions for the management of ASM after OHS besides monitoring the frequency of ASM, which can usually be achieved using postoperative echocardiography. Furthermore, to our knowledge, there was no published study that investigated whether ASM is a consequence of pericardial constriction.
Righetti et al.12) reported that ASM is related to ischemic injury to the septum during CBS. However, other subsequent studies have demonstrated that ischemic injury is an unreliable mechanism for ASM.13)14) Further, our results suggest that ischemic injury is not related to ASM. An ischemic injury to the septum would result in a decrease in septal thickness; however, our data indicated intact septal thickness after surgery in patients with ASM. Furthermore circumferential and global strains, which are more sensitive tools for detecting ischemic injury of the myocardium,15)16) did not change preoperatively and postoperatively in both groups. LV ejection fraction and systolic mitral annular velocity, which is a good tool for systolic function assessment in patients with ASM,17) was similar in both groups.
The other possible explanation is the change of the position or mobility of the heart within the chest. Moreover, ASM is a typical finding associated with the congenital absence of the pericardium18) or pericardiectomy.7) De Nardo et al.19) reported an increased anterior motion of the entire heart because of pericardiotomy, and Wranne et al.20) demonstrated the restriction of the right ventricular contraction from the chest walls using transesophageal echocardiography during surgery. Similarly, our data showed that systolic VRad of the antero-septum and anterior wall decreased during systole after OHS in patients with ASM. This finding reflects a decreased inward motion of the antero-septum compared to other segments of the LV myocardium and thus, indicates exaggerating interventricular septal motion. However still, the reason for the reduction in systolic VRad in the antero-septum and anterior wall remains unclear. We hypothesized that subtle conduction disturbance (transient or not) after cardiac surgery21)22) is a possible explanation for the systolic VRad reduction. However, postoperative electrocardiograms did not indicate left or right bundle branch, or any fascicular block. In addition, it might be just the results of ASM, not cause, which is very important limitation of this study. Nevertheless, we could confirm that ASM was not caused by myocardial ischemia.
According to a large scale review of 3923 cases by Reynolds et al.,5) the incidence of ASM was 40%, and valve surgery was more likely to cause ASM compared to CBS. In the present study, the incidence of ASM was 74%, almost two-fold what was previously reported, but we did not observe an association between the type of surgery and the occurrence of ASM. The same mechanism may be responsible for ASM in both CBS and HVS.
In the literature, it is described that ASM occurs immediately after surgery and usually tends to resolve with time, although it can persist indefinitely in some patients.23) In consensus with this statement, our data showed ASM disappeared over time in most patients without clinically detectable pericardial constriction. Only 3 patients in our study sample had clinically significant transient constrictions probably because of the use of steroids; in 2 of these patients, ASM disappeared over time.
In summary, ASM is frequent after OHS, however, ASM does not seem to have any clinical significance and will likely disappear over time. Further, we demonstrated that decreased postoperative systolic VRad of the antero-septum and anterior wall is associated with the occurrence of ASM.
Echocardiographic views are relatively poorer after HVS than those after CBS. Therefore, the occurrence of ASM after HVS might be underestimated. Although data from all the patients were available immediately after the operation, data from the 3--6-month and 1-year follow-up visits were only available for 40--45% of the patients each group. In addition, we performed VVI analysis before and immediately after surgery in only a limited number of patients (approximately 40 patients), and we were unable to identify reversal of systolic VRad of the antero-septum and anterior wall when ASM disappeared. Lastly, we usually use diuretics peri-OHS; thus, some patients with constrictive physiology may not have been revealed. However, this limitation may not be clinically relevant, even in patients with ASM, because the majority of study patients did not require prolonged diuretic administration after OHS.
Even though ASM was common in patients immediately after cardiac surgery, it disappeared over time without causing clinically detectable pericardial constriction. Furthermore, ASM might not be caused by myocardial ischemia, but we demonstrated that a decreased postoperative systolic VRad of the antero-septum and anterior wall is associated with ASM after cardiac surgery.
Figures and Tables
Fig. 1
Abnormal interventricular septal motion after open heart surgery detected using M-mode echocardiography. Note that the interventricular septum is moving anteriorly with systole (yellow arrows).
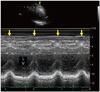
Fig. 3
Postoperative velocity vector image strain analysis. A and B: The circumferential strain analysis of ASM- and ASM+. C and D: The changes of radial velocity of ASM- and ASM+. ASM: abnormal interventricular septal motion, VRad: radial velocity, AS: antero-septum, AW: anterior wall, AL: anterolateral, IL: inferolateral, I: inferior, IS: inferoseptum.

Table 2
Pre and post-operative echocardiographic parameters
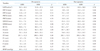
ASM: abnormal interventricular septal motion, LAVI: left atrial volume index, SWT: septal wall thickness, d: diastolic, s: systolic, PWT: posterior wall thickness, LVEDD: left ventricular end diastolic dimension, LVESD: left ventricular end systolic dimension, EF: ejection fraction, E: mitral early diastolic filling velocity, A: mitral late diastolic filling velocity, DT: deceleration time, E': early mitral annular velocity, A': late mitral annular velocity, S': systolic mitral annular velocity, RVSP: right ventricular systolic pressure
Acknowledgements
This study was supported by the Korean National Research Foundation and funded with a grant from the Korean Government (MEST) (No. 2012027176).
References
1. Hurrell DG, Nishimura RA, Higano ST, Appleton CP, Danielson GK, Holmes DR Jr, Tajik AJ. Value of dynamic respiratory changes in left and right ventricular pressures for the diagnosis of constrictive pericarditis. Circulation. 1996; 93:2007–2013.

2. Burstow DJ, Oh JK, Bailey KR, Seward JB, Tajik AJ. Cardiac tamponade: characteristic Doppler observations. Mayo Clin Proc. 1989; 64:312–324.

3. Ling LH, Oh JK, Breen JF, Schaff HV, Danielson GK, Mahoney DW, Seward JB, Tajik AJ. Calcific constrictive pericarditis: is it still with us? Ann Intern Med. 2000; 132:444–450.

4. Schroeder E, Marchandise B, Schoevaerdts JC, Kremer R. Paradoxical ventricular septal motion after cardiac surgery. Analysis of M-mode echocardiograms and follow-up in 324 patients. Acta Cardiol. 1985; 40:315–324.
5. Reynolds HR, Tunick PA, Grossi EA, Dilmanian H, Colvin SB, Kronzon I. Paradoxical septal motion after cardiac surgery: a review of 3,292 cases. Clin Cardiol. 2007; 30:621–623.

6. Wigle ED, Sasson Z, Henderson MA, Ruddy TD, Fulop J, Rakowski H, Williams WG. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985; 28:1–83.

7. Eslami B, Roitman D, Karp RB, Sheffield LT. The echocardiogram after pericardiectomy. Jpn Heart J. 1979; 20:1–5.

8. Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, Armstrong WF. An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol. 1985; 5:918–927.

9. Zoneraich S, Zoneraich O, Rhee JJ. Echocardiographic evaluation of septal motion in patients with artificial pacemakers: vectorcardiographic correlations. Am Heart J. 1977; 93:596–602.

10. Abbasi AS, Eber LM, MacAlpin RN, Kattus AA. Paradoxical motion of interventricular septum in left bundle branch block. Circulation. 1974; 49:423–427.

11. Hishida H, Sotobata I, Koike Y, Okumura M, Mizuno Y. Echocardiographic patterns of ventricular contraction in the Wolff-Parkinson-White Syndrome. Circulation. 1976; 54:567–570.

12. Righetti A, Crawford MH, O'rourke RA, Schelbert H, Daily PO, Ross J Jr. Interventricular septal motion and left ventricular function after coronary bypass surgery: evaluation with echocardiography and radionuclide angiography. Am J Cardiol. 1977; 39:372–377.

13. Joshi SB, Salah AK, Mendoza DD, Goldstein SA, Fuisz AR, Lindsay J. Mechanism of paradoxical ventricular septal motion after coronary artery bypass grafting. Am J Cardiol. 2009; 103:212–215.

14. Choi SH, Choi SI, Chun EJ, Chang HJ, Park KH, Lim C, Kim SJ, Kang JW, Lim TH. Abnormal motion of the interventricular septum after coronary artery bypass graft surgery: comprehensive evaluation with MR imaging. Korean J Radiol. 2010; 11:627–631.

15. Kido T, Nagao M, Kido T, Kurata A, Miyagawa M, Ogimoto A, Mochizuki T. Stress/rest circumferential strain in non-ischemia, ischemia, and infarction--quantification by 3 Tesla tagged magnetic resonance imaging. Circ J. 2013; 77:1235–1241.

16. Grenne B, Eek C, Sjøli B, Dahlslett T, Hol PK, Orn S, Skulstad H, Smiseth OA, Edvardsen T, Brunvand H. Mean strain throughout the heart cycle by longitudinal two-dimensional speckle-tracking echocardiography enables early prediction of infarct size. J Am Soc Echocardiogr. 2011; 24:1118–1125.

17. Silva JA, Khuri B, Barbee W, Fontenot D, Cheirif J. Systolic excursion of the mitral annulus to assess septal function in paradoxic septal motion. Am Heart J. 1996; 131:138–145.

18. Connolly HM, Click RL, Schattenberg TT, Seward JB, Tajik AJ. Congenital absence of the pericardium: echocardiography as a diagnostic tool. J Am Soc Echocardiogr. 1995; 8:87–92.

19. De Nardo D, Caretta Q, Mercanti C, Alessandri N, Scibilia G, Chiavarelli R, Antolini M, Pitucco G, Caputo V, Marino B. Effects of uncomplicated coronary artery bypass graft surgery on global and regional left ventricular function at rest. Study by equilibrium radionuclide angiocardiography. Cardiology. 1989; 76:285–292.

20. Wranne B, Pinto FJ, Siegel LC, Miller DC, Schnittger I. Abnormal postoperative interventricular motion: new intraoperative transesophageal echocardiographic evidence supports a novel hypothesis. Am Heart J. 1993; 126:161–167.

21. Flack JE 3rd, Hafer J, Engelman RM, Rousou JA, Deaton DW, Pekow P. Effect of normothermic blood cardioplegia on postoperative conduction abnormalities and supraventricular arrhythmias. Circulation. 1992; 86:5 Suppl. II385–II392.
22. Emlein G, Huang SK, Pires LA, Rofino K, Okike ON, Vander Salm TJ. Prolonged bradyarrhythmias after isolated coronary artery bypass graft surgery. Am Heart J. 1993; 126:1084–1090.

23. Otto CM. Textbook of clinical echocardiography. 3rd ed. Philadelphia: Elsevier Saunders;2004. p. 154.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download