1. Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010; 23:351–369. quiz 453-5.

2. Taber LA, Yang M, Podszus WW. Mechanics of ventricular torsion. J Biomech. 1996; 29:745–752.

3. Sengupta PP, Krishnamoorthy VK, Korinek J, Narula J, Vannan MA, Lester SJ, Tajik JA, Seward JB, Khandheria BK, Belohlavek M. Left ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr. 2007; 20:539–551.

4. Torrent-Guasp F, Ballester M, Buckberg GD, Carreras F, Flotats A, Carrió I, Ferreira A, Samuels LE, Narula J. Spatial orientation of the ventricular muscle band: physiologic contribution and surgical implications. J Thorac Cardiovasc Surg. 2001; 122:389–392.

5. Nakatani S. Left ventricular rotation and twist: why should we learn? J Cardiovasc Ultrasound. 2011; 19:1–6.

6. Buckberg GD. Basic science review: the helix and the heart. J Thorac Cardiovasc Surg. 2002; 124:863–883.

7. Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008; 1:366–376.
8. Esch BT, Warburton DE. Left ventricular torsion and recoil: implications for exercise performance and cardiovascular disease. J Appl Physiol (1985). 2009; 106:362–369.

9. Hansen DE, Daughters GT 2nd, Alderman EL, Stinson EB, Baldwin JC, Miller DC. Effect of acute human cardiac allograft rejection on left ventricular systolic torsion and diastolic recoil measured by intramyocardial markers. Circulation. 1987; 76:998–1008.

10. Ingels NB Jr, Daughters GT 2nd, Stinson EB, Alderman EL. Measurement of midwall myocardial dynamics in intact man by radiography of surgically implanted markers. Circulation. 1975; 52:859–867.

11. Gustafsson U, Lindqvist P, Mörner S, Waldenström A. Assessment of regional rotation patterns improves the understanding of the systolic and diastolic left ventricular function: an echocardiographic speckle-tracking study in healthy individuals. Eur J Echocardiogr. 2009; 10:56–61.

12. Rüssel IK, Götte MJ, Kuijer JP, Marcus JT. Regional assessment of left ventricular torsion by CMR tagging. J Cardiovasc Magn Reson. 2008; 10:26.

13. Garot J, Pascal O, Diébold B, Derumeaux G, Gerber BL, Dubois-Randé JL, Lima JA, Guéret P. Alterations of systolic left ventricular twist after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2002; 282:H357–H362.
14. Helle-Valle T, Remme EW, Lyseggen E, Pettersen E, Vartdal T, Opdahl A, Smith HJ, Osman NF, Ihlen H, Edvardsen T, Smiseth OA. Clinical assessment of left ventricular rotation and strain: a novel approach for quantification of function in infarcted myocardium and its border zones. Am J Physiol Heart Circ Physiol. 2009; 297:H257–H267.

15. Notomi Y, Lysyansky P, Setser RM, Shiota T, Popović ZB, Martin-Miklovic MG, Weaver JA, Oryszak SJ, Greenberg NL, White RD, Thomas JD. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol. 2005; 45:2034–2041.

16. Arab-Baferani Z, Mokhtari-Dizaji M, Roshanali F. Extraction of left-ventricular torsion angle from the long-axis view by block-matching algorithm: comparison with the short-axis view. Ultrasonics. 2013; 53:552–560.

17. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998; 97:1837–1847.

18. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002; 18:539–542.

19. Boukerroui D, Noble JA, Brady M. Velocity estimation in ultrasound images: a block matching approach. Inf Process Med Imaging. 2003; 18:586–598.

20. Chen EJ, Jenkins WK, O'Brien WD Jr. The impact of various imaging parameters on ultrasonic displacement and velocity estimates. IEEE Trans Ultrason Ferroelectr Freq Control. 1994; 41:293–301.

21. Chadwick RS. Mechanics of the left ventricle. Biophys J. 1982; 39:279–288.

22. Arts T, Veenstra PC, Reneman RS. Epicardial deformation and left ventricular wall mechanisms during ejection in the dog. Am J Physiol. 1982; 243:H379–H390.

23. Guccione JM, Moonly SM, Moustakidis P, Costa KD, Moulton MJ, Ratcliffe MB, Pasque MK. Mechanism underlying mechanical dysfunction in the border zone of left ventricular aneurysm: a finite element model study. Ann Thorac Surg. 2001; 71:654–662.

24. Guccione JM, Moonly SM, Wallace AW, Ratcliffe MB. Residual stress produced by ventricular volume reduction surgery has little effect on ventricular function and mechanics: a finite element model study. J Thorac Cardiovasc Surg. 2001; 122:592–599.

25. Yeung F, Levinson SF, Fu D, Parker KJ. Feature-adaptive motion tracking of ultrasound image sequences using a deformable mesh. IEEE Trans Med Imaging. 1998; 17:945–956.

26. Urbano Moral JA, Arias Godinez JA, Maron MS, Malik R, Eagan JE, Patel AR, Pandian NG. Left ventricular twist mechanics in hypertrophic cardiomyopathy assessed by three-dimensional speckle tracking echocardiography. Am J Cardiol. 2011; 108:1788–1795.

 = (a⊥z, az) and
= (a⊥z, az) and 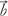 = (b⊥z, bz) are position vectors in long-axis view. Thus, the rotation angle in long-axis view [χ(t)] is obtained from equation 5:
= (b⊥z, bz) are position vectors in long-axis view. Thus, the rotation angle in long-axis view [χ(t)] is obtained from equation 5:
 = (ax, ay) and
= (ax, ay) and 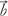 = (bx, by) are position vectors in short-axis view. Thus, the rotation angle in short-axis view [θ(t)] is obtained from equation 6:
= (bx, by) are position vectors in short-axis view. Thus, the rotation angle in short-axis view [θ(t)] is obtained from equation 6:
 = (ax, ay, az) and
= (ax, ay, az) and 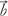 = (bx, by, bz) are position vectors in short-axis view. Thus, the 3-dimensional angle of trajectory [Φ(t)] is obtained from equation 7:
= (bx, by, bz) are position vectors in short-axis view. Thus, the 3-dimensional angle of trajectory [Φ(t)] is obtained from equation 7:




 PDF
PDF ePub
ePub Citation
Citation Print
Print











 XML Download
XML Download