Abstract
Differentiation of normal from abnormal findings is critical in echocardiography. Anatomic variants occurring in normal cardiac developments often simulate pathologic entities. This review focuses on the differential diagnosis of normal anatomic structures from pathologic ones in echocardiography.
Anatomic variants are particularly common in the right atrium (RA). During cardiac development, embryonic sinus venosus fuses with trabecular RA appendage.1) The right sinus valve of embryonic sinus venosus normally regresses forming the crista terminalis and the Eustachian valve. Such regression process is highly variable. Incomplete regression results a spectrum of vestiges, such as Chiari network, Eustachian valve of inferior vena cava (IVC), the Thebesian valve of the coronary sinus, and a prominent crista terminalis.2)
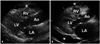
The Eustachian valve is a remnant of embryonic valve of IVC, which directs oxygen-rich vena caval return toward interatrial septum and left atrium (LA) in fetal heart. Eustachian valve appears as crescent-like fold of variable size at posterior margin of IVC (Fig. 1). In echocardiography, leaf-like linear structure is shown at the junction of IVC and RA. Right ventricular (RV) inflow view, subxiphoid view, and transesophageal echocardiography (TEE) is diagnostic because such windows can visualize both Eustachian valve and IVC in the same imaging plane. Occasionally, prominent Eustachian valve appears to divide RA into two chambers making apparent cor triatriatum dexter (Fig. 2). Such condition is hemodynamically insignificant in most adults because the septation by Eustachian valve is generally incomplete.
Chiari network is a thin, web-like fenestrated membrane that attaches along the ridge connecting vena cavae and interatrial septum. It is found in 2-3% of normal heart at autopsy.2) In echocardiography, Chiari network appears as free floating curvilinear structure that waves with blood flow in RA (Fig. 3). Chiari network is thought to a variant of Eustachian valve. A part of Chiari network arises from the orifice of IVC like Eustachian valve, but Chiari network is much more mobile and thinner. In echocardiography, Chiari network may be confused for tricuspid vegetation, flail tricuspid valve, free RA thrombus, and pedunculated tumors.3) Careful tracing to identify its attachment to the orifice of IVC makes a differential diagnosis. Chiari network has little clinical significance, but it might cause trouble during percutaneous procedures. The cases of entrapment of right-heart catheters, or entanglement and herniation into the LA by atrial septal defect occluding device have been reported.4)5)
Crista terminalis is a well-defined fibromuscular ridge separating a smooth sinus venarum and trabeculated RA.6) Externally, it corresponds to the sulcus terminalis, and internally, it extends from the superior vena cava (SVC) to IVC along the lateral RA wall. Embryologically, crista terminalis develops from the septum spurium, which corresponds to the fused boundary between embryonic sinus venosus and RA proper.1) Prominent crista terminalis may be confused for RA tumor on transthoracic echocardiography (Fig. 4).7) Echocardiographic findings suggestive of prominent crista terminalis instead of tumor are as followings: a nodular mass of similar echogenicity with adjacent myocardium; the location of posterolateral wall of RA near the SVC, which corresponds to the course of crista terminalis connecting the SVC and IVC; the phasic change in size becoming thicker or larger during atrial systole.8) Bicaval view of TEE best visualizes the crista terminalis.
Thrombi in the RA may mimic anatomic variants (Fig. 5). Clinical settings would help a diagnosis. The presence of atrial arrhythmia including atrial fibrillation, low flow status including RV failure, and the presence of foreign body favor the likelihood of thrombi. Thromboemboli-in-transit that arises in low extremity vein may be found in RA. Migrating free thrombi appears highly mobile snake-like structure mimicking Chiari network. However, migrating thrombi is thicker than Chiari network, and the end of thrombi is not fixed around the IVC orifice.
Lastly, RA tumors are needed to be differentiated from anatomic variants. Myxoma is the most common primary tumor occurring in RA. Echocardiographically, myxoma appears as a globular or spherical mass with friable surface and heterogenous internal echogenicity. Myxomas typically arise from the interatrial septum around the fossa ovalis.2) Metastatic tumors including hepatoma, renal cell cancer, and sarcoma from pelvic organs reaching RA through IVC can be seen in echocardiography. Careful tracing the origin of mass will give a clue for differential diagnosis from benign anatomic variants.
Heavy trabeculation, prominent or redundant papillary muscle, and moderator band may be normally seen in the RV. They need to be differentiated from pathologic entities such as primary and metastatic tumors, thrombi, and vegetations.
Multiple trabeculation is a characteristic of RV. The pattern of trabeculation is highly variable and the exaggeration of normal trabeculation might be confused for cardiomyopathy. True pathologic hypertrabeculation occurring in developmental arrest of RV myocardium is rare, and it is often accompanied by the other congenital heart diseases including tricuspid valve anomaly, atrial or ventricular septal defect, and left ventricular (LV) non-compaction.
Moderator band is a prominent trabeculation of RV extending from the base of anterior papillary muscle to the interventricular septum, which contains right bundle branch. It is present in the majority of normal adults but tremendous individual variations are observed in thickness and shape. In echocardiography, moderator band is a thick echo-dense band-like structure across the RV cavity and connects the lower interventricular septum and the anterior papillary muscle (Fig. 6).
RV may be involved by true pathologic lesions. Thrombi may be present in RV failure, eosinophilic endocarditis, myocardial infarction involving right coronary artery, and rarely RV cardiomyopathy with aneurysm. As thrombi involving RV often occur at distal RV, plenty of trabeculation normally present in the distal RV cavity may prevent to identify a small thrombi. Careful evaluation with multiple off-axis imaging planes is needed for the suspected case with RV dysfunction. RV may be involved a tumorous condition including rhabdomyoma and metastatic tumors. Tumors may cause RV failure by direct invasion and myocardial replacement with tumor infiltration, cavity obliteration or limiting tricuspid motion (Fig. 7).9)
LV bands or false tendons are fibromuscular structures crossing the LV cavity. LV bands may pass between papillary muscles, from papillary muscle to the ventricular septum, between free walls, or from free wall to interventricular septum, in contrary to true chordae tendineae connecting papillary muscle and mitral valve leaflets.10)11) False tendon is found up to 55% in normal hearts by autopsy study.12) In echocardiography, LV bands appear as string-like thin bands passing LV cavity (Fig. 8), which may be transverse, longitudinal, or sagittal, and single or multiple. The location, direction, length and thickness of LV bands may vary depending on their embryonic origin of inner cardiac muscle layer and contents. Muscular bands become shorter and thicker in systole, and vice versa in diastole. Fibrous bands become straight and taut in diastole, and vice versa in systole.13) Off-axis images demonstrating the overall length of bands, normal LV structures on both ends, and constant motion during cardiac cycle are the key features.11) False tendon located near LV apex may be confused for mural thrombus particularly in images of true LV apex being not completely visualized (Fig. 8B).
Papillary muscles vary in shape, thickness, and the location in LV wall. More than one belly is observed in up to 50% of normal hearts.14) Accessory papillary muscle may be confused for pathologic structures such as LV thrombus or papillary muscle tumors when it arises from an unusual location. The presence of LV band is strongly indicative of the accessory papillary muscle instead of pathologic entity.15) Normally contractile adjacent LV wall help to exclude the mural thrombi. Papillary muscle variants in architecture and location have peculiar clinical significance in hypertrophic cardiomyopathy. Anterior displacement of hypertrophied papillary muscle is known to accentuate the resting trans-LV outflow tract pressure gradients.16)17)
Atrial septal aneurysm (ASA) is found in 1% of adults at autopsy.18) An excursion of > 10 mm beyond the plane of interatrial septum is recognized as ASA,19) although such cut-off value is arbitrary. ASA may involve only the region of fossa ovalis, or the entire interatrial septum.20) Frequent association with atrial septal defect, patent foramen ovale (PFO), mitral valve prolapse, Marfan syndrome suggests that ASA is congenital malformation with genetic background.19) Longstanding elevation of atrial pressure may contribute to ASA formation, inducing a septum to bulge toward the lower pressure chamber. In echocardiography, redundant interatrial septum bulges beyond the atrial septal plane (Fig. 9). Phasic oscillation along the cardiac or respiratory cycle is common. Prominent ASA may appear as cystic mass in long axis views, but diagnosis is rarely difficult particularly with the aid of TEE. ASA is known to be associated with atrial arrhythmia and ischemic stroke.21)22) ASA is frequently accompanied by PFO causing embolic events, and ASA itself was known as an independent predictor of cryptogenic stroke.22)
The term of lipomatous hypertrophy refers the condition of prominent thickening of interatrial septum, usually > 2 cm, caused by excessive fatty infiltration. It is sometimes misclassified as benign tumor, however, it actually represents the fat-filled extracardiac spaces which is not encapsulated unlike true lipoma. Echocardiographic diagnosis is made when a marked atrial septal thickening > 15-20 mm in the absence of any other explanation for the abnormal thickening.23-25) The region of fossa ovalis is typically spared, which makes a characteristic dumbbell- or hour glass-shaped lesion. Subcostal window can be best used (Fig. 10). The superior and inferior "mass" is corresponds to the fat-filled groove between atria (Waterston's groove) and ventricles (inferior pyramidal space), respectively,26)27) that is, fatty mass of lipomatous hypertrophy is contiguous with epicardial fat pads. It is understandable that patients with lipomatous hypertrophy tend to have heavy pericardial and periaortic fat infiltration.24) Lesser degree of atrial septal thickening can occur in amyloidosis, tumors, and a surgical patch covering repaired atrial septal defect.2)24) Typical bi-lobed appearance with sparing of fossa ovalis, and clinical information for a systemic illness would guide a diagnosis. Otherwise, computed tomography (CT) and magnetic resonance imaging are useful to differentiate fatty infiltration.24)28) Lipomatous hypertrophy is generally benign condition and asymptomatic. However, the blood flow obstruction of SVC and coronary sinus, intra-atrial conduction disturbance, supraventricular arrhythmia, syncope, and even sudden death had been reported.24)29)
Lambl's excrescences are fine filamentous lesions of valvular leaflets.30) It increases with age and is considered as a degenerative change on the surface of leaflets due to mechanical wear and tear. Aortic valve is commonly involved. Fine strands have acellular connective tissue cores with some elastic fibers.31) Multiple adjacent excrescences may stick together and grow up to large, complex form called "giant Lambl's excrescence". Whether the excrescences may serve as a nidus for bacterial growth or cause a systemic embolism is controversial.2)32-34) In echocardiography, it appears as very thin, delicate, lint-like mobile threads arising from the free borders or ventricular surfaces of aortic leaflets (Fig. 11). It may be multiple and several centimeters long. Improving image quality increases to find this lesion. The echocardiographic significance of Lambl's excrescences lies in the differential diagnosis from the vegetation of infective endocarditis. It is challenging in most cases, and a diagnostic decision making often depends on clinical settings.
Papillary fibroelastoma is a benign avascular tumor arising from the normal endocardium.35)36) It can occur anywhere in the heart, but most frequently arise from valvular endocardium.37) Most papillary fibroelastoma are found in elderly, and it may be a hamartoma developing in a degenerative wear-and-tear process.2) Characteristic numerous gelatinous papillary fronds of tumor surface consist of dense connective tissue core covered by endothelium.38) In echocardiography, a small mobile tumor with fine frond-like surface attaches to the downstream side of the valve by a small stalk (Fig. 12).30) Surgical resection is needed as it may cause a systemic embolism. It is challenging to differentiate a papillary fibroelastoma from giant Lambl's excrescence, as they are similar both echocardiographically and pathologically. These two entities might belong to a pathologic spectrum sharing several features.39)
Pericardium is a flask-shaped potential space formed between visceral and parietal pericardium.40) It normally contains small amount of fluid to lubricate cardiac movement. The visceral pericardium covers the surface of heart and the proximal segments of great vessels, and then, reflects as the inner lining layer of parietal pericardium. At the site of reflections, a network of pericardial sinuses and recesses are formed. Such pericardial sinuses may appear separate at a cross-sectional image, however, they are mutually connected each other. Free pericardial fluid distributed within the transverse sinus and several recesses might be confused for a periannular abscess in transthoracic and TEE (Fig. 14). Diagnosis is particularly complicated in the febrile patients of post-operative course because the tissue edema and fluid collection due to inflammatory or procedural causes mimic the true pathologic process.
Epicardial adipose tissue often appears as echo-free space mimicking pericardial effusion in echocardiography. Most adipose tissue distributes in atrio-ventricular or interventricular groove. Excessive fatty infiltration tends to occur in old, obese, and diabetic patients, particularly in women.
Echocardiographic differentiation of adipose tissue from fluid is based on echogenicity, texture, mobility, and location.2) Inflammatory or bloody effusion may be extremely thick to show high and heterogenous echogenicity. With exception of loculated effusion, free pericardial fluid accumulates on dependent region, usually posterior of left in supine position. Anteriorly located echo-free space is likely to be epicardial fat deposition rather than pericardial effusion (Fig. 15). Mobility of the adjacent tissue is another clue to differentiation. As adipose tissue is less mobile than pericardial fluid, surrounding epicardial and pericardial layer move less freely in case of fatty infiltration. Heart moves like "swinging" in moderate amount of pericardial effusion, but not in fat-filled space. Lastly, adipose tissue is more echogenic and lobulated than fluid with homogenous echogenicity. CT imaging can definitely differentiate epicardial adipose tissue from fluid collection in complicated case.
The differential diagnosis of "mass" occurring heart is often challenging although the imaging quality and techniques are quite beautiful in current echocardiography. Knowledge about anatomic variants would guide to proper interpretation of echocardiographic findings. Previous images should be reviewed carefully whenever it is available. Short-term repetition of imaging on the mass is extremely helpful to diagnosis and not to miss the critical change of the pathologic entities.
Figures and Tables
Fig. 1
Eustachian valve. Note the leaf-like linear structure (arrowhead) at the junction of IVC RA in four chambers (A), right ventricular inflow (B) and bicaval view of transesophageal echocardiography (C). Ao: ascending aorta, IAS: interatrial septum, IVC: inferior vena cava, LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.

Fig. 2
Prominent Eustachian valve (arrowhead) making an apparent cor triatriatum dexter. LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
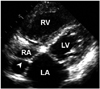
Fig. 3
A: Chiari network (arrowheads) is the delicate freely mobile membranous structure in the RA. B: RV inflow view shows its close relationship with IVC. IVC: inferior vena cava, LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
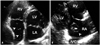
Fig. 4
Crista terminalis (arrowhead) appears as the mass arising from the posterior wall of RA in four chamber view (A), but it is well visualized as muscular ridge near SVC in transesophageal echocardiography (B). EV: Eustachian valve, IVC: inferior vena cava, LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle, SVC: superior vena cava.
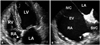
Fig. 5
A: Thrombi of RA (arrowheads) in parasternal short axis view. B: Computed tomography imaging of the same patient. Ao: ascending aorta, LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
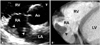
Fig. 6
Normal variants of RV. A: Moderator band (arrowhead). B: Prominent papillary muscle. C: Hypertrabeculation (asterisk). IVS: interventricular septum, LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
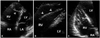
Fig. 7
Pathologic lesions involving RV. A: Thrombus (arrowheads) attached to chordae tendineae and the wall of RV with significant dilation and impaired contractility. B: Irregular thickening of RV free wall (arrowheads) due to metastatic lung cancer. Note the pericardial effusion around the RV. LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
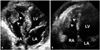
Fig. 8
Normal LV variants. A: Fibrous LV band (arrowhead) pass the LV cavity with transverse direction. B: Muscular LV band (arrowhead). C: Apically located papillary muscle (arrowheads) with chordae tendineae. LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
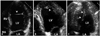
Fig. 9
A: Atrial septal aneurysm (arrowhead) in four chamber view. B: It may show tissue drop mimicking atrial septal defect. C: The degree of excursion (a) from the atrial septal plane (b) is used to quantify the size of atrial septal aneurysm. LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.

Fig. 10
Lipomatous hypertrophy of atrial septum in subxyphoid view. Marked fatty infiltration of the superior and inferior region (asterisks) of atrial septum with central sparing result dumbbell-shaped appearance. arrowhead: interatrial septum, LA: left atrium, RA: right atrium.
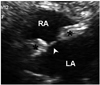
Fig. 11
Lambl's excrescence in parasternal long (A) and short (B) axis view. Threadlike fronds (arrowhead) on the sites of aortic valve closure are flapping along the cardiac cycle. Ao: ascending aorta, AV: aortic valve, LV: left ventricle.
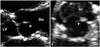
Fig. 12
Papillary fibroelastoma (arrowhead) in aortic (A) and tricuspid valve (B). It appears jelly-like small nodular mass with short stalk. Ao: ascending aorta, LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
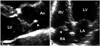
Fig. 13
A: Vegetation (arrowhead) is formed on the path of regurgitant jet. B: The inflammation and leaflet destruction with significant regurgitation are additional finding. Ao: ascending aorta, LV: left ventricle.
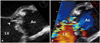
Fig. 14
Periaortic echo-free space. A: Transverse pericardial sinus (asterisk) is normally seen at short axis view. B: Periannular abscess (asterisk) due to infective endocarditis in a patient with prosthetic aortic valve. Ao: ascending aorta, AVR: aortic valve replacement, LA: left atrium.
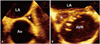
Fig. 15
Pericardial echo-free space. A: Epicardial fat (asterisk) showing anterior location and granular texture. B: Pericardial effusion (asterisk) showing clear echo-free space with posterior-dominant location and freely moving heart. Ao: ascending aorta, LA: left atrium, LV: left ventricle, RV: right ventricle.
References
1. Schoenwolf GC, Bleyl SB. Larsen's human embryology. 4th ed. Philadelphia: Churchill Livingstone/Elsevier;2009.
2. Weyman AE. Principles and practice of echocardiography. 2nd ed. Philadelphia: Lippincott Williams & Wilkins;1994.
3. Werner JA, Cheitlin MD, Gross BW, Speck SM, Ivey TD. Echocardiographic appearance of the Chiari network: differentiation from right-heart pathology. Circulation. 1981; 63:1104–1109.

4. Cooke JC, Gelman JS, Harper RW. Chiari network entanglement and herniation into the left atrium by an atrial septal defect occluder device. J Am Soc Echocardiogr. 1999; 12:601–603.

5. Goldschlager A, Goldschlager N, Brewster H, Kaplan J. Catheter entrapment in a Chiari network involving an atrial septal defect. Chest. 1972; 62:345–346.

6. Loukas M, Tubbs RS, Tongson JM, Polepalli S, Curry B, Jordan R, Wagner T. The clinical anatomy of the crista terminalis, pectinate muscles and the teniae sagittalis. Ann Anat. 2008; 190:81–87.

7. Mirowitz SA, Gutierrez FR. Fibromuscular elements of the right atrium: pseudomass at MR imaging. Radiology. 1992; 182:231–233.

8. Salustri A, Bakir S, Sana A, Lange P, Al Mahmeed WA. Prominent crista terminalis mimicking a right atrial mass: case report. Cardiovasc Ultrasound. 2010; 8:47.

9. Ports TA, Schiller NB, Strunk BL. Echocardiography of right ventricular tumors. Circulation. 1977; 56:439–447.

10. Nishimura T, Kondo M, Umadome H, Shimono Y. Echocardiographic features of the false tendons in the left ventricle. Am J Cardiol. 1981; 48:177–183.
11. Keren A, Billingham ME, Popp RL. Echocardiographic recognition and implications of ventricular hypertrophic trabeculations and aberrant bands. Circulation. 1984; 70:836–842.

12. Luetmer PH, Edwards WD, Seward JB, Tajik AJ. Incidence and distribution of left ventricular false tendons: an autopsy study of 483 normal human hearts. J Am Coll Cardiol. 1986; 8:179–183.

13. George A, Parameswaran A, Nekkanti R, Lurito K, Movahed A. Normal anatomic variants on transthoracic echocardiogram. Echocardiography. 2009; 26:1109–1117.

14. Victor S, Nayak VM. Variations in the papillary muscles of the normal mitral valve and their surgical relevance. J Card Surg. 1995; 10:597–607.

15. Misra S, Koshy T, Pal S. Echo rounds: false tendons and accessory papillary muscle in the left ventricle. Anesth Analg. 2011; 113:1016–1018.
16. Kwon DH, Setser RM, Thamilarasan M, Popovic ZV, Smedira NG, Schoenhagen P, Garcia MJ, Lever HM, Desai MY. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart. 2008; 94:1295–1301.

17. Harrigan CJ, Appelbaum E, Maron BJ, Buros JL, Gibson CM, Lesser JR, Udelson JE, Manning WJ, Maron MS. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol. 2008; 101:668–673.

18. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978; 102:62–65.
19. Mügge A, Daniel WG, Angermann C, Spes C, Khandheria BK, Kronzon I, Freedberg RS, Keren A, Denning K, Engberding R, et al. Atrial septal aneurysm in adult patients. A multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995; 91:2785–2792.
20. Hanley PC, Tajik AJ, Hynes JK, Edwards WD, Reeder GS, Hagler DJ, Seward JB. Diagnosis and classification of atrial septal aneurysm by two-dimensional echocardiography: report of 80 consecutive cases. J Am Coll Cardiol. 1985; 6:1370–1382.

21. Ong LS, Nanda NC, Falkoff MD, Barold SS. Interatrial septal aneurysm, systolic click and atrial tachyarrhythmia--a new syndrome? Ultrasound Med Biol. 1982; 8:691–693.

22. Mattioli AV, Aquilina M, Oldani A, Longhini C, Mattioli G. Atrial septal aneurysm as a cardioembolic source in adult patients with stroke and normal carotid arteries. A multicentre study. Eur Heart J. 2001; 22:261–268.

23. Page DL. Lipomatous hypertrophy of the cardiac interatrial septum: its development and probable clinical significance. Hum Pathol. 1970; 1:151–163.

24. Heyer CM, Kagel T, Lemburg SP, Bauer TT, Nicolas V. Lipomatous hypertrophy of the interatrial septum: a prospective study of incidence, imaging findings, and clinical symptoms. Chest. 2003; 124:2068–2073.
25. Fyke FE 3rd, Tajik AJ, Edwards WD, Seward JB. Diagnosis of lipomatous hypertrophy of the atrial septum by two-dimensional echocardiography. J Am Coll Cardiol. 1983; 1:1352–1357.

26. Cunningham KS, Veinot JP, Feindel CM, Butany J. Fatty lesions of the atria and interatrial septum. Hum Pathol. 2006; 37:1245–1251.

27. Shirani J, Roberts WC. Clinical, electrocardiographic and morphologic features of massive fatty deposits ("lipomatous hypertrophy") in the atrial septum. J Am Coll Cardiol. 1993; 22:226–238.

28. Stephant E, Barthelet M, Leroux PY, Revel D. Images in cardiovascular medicine. Lipomatous hypertrophy of the interventricular septum: echocardiography, cardiac magnetic resonance, and multidetector computerized tomography imaging. Circulation. 2008; 118:e71–e72.
29. Riva L, Banfi C, Gaeta R, Vigano M. Lipomatous hypertrophy of the interatrial septum. Description of a clinical case and literature review. Minerva Cardioangiol. 2006; 54:789–792.
30. Armstrong WF, Ryan T. Feigenbaum's echocardiography. 7th ed. Philadelphia: Lippincott Williams & Wilkins;2010.
31. Magarey FR. On the mode of formation of Lambl's excrescences and their relation to chronic thickening of the mitral valve. J Pathol Bacteriol. 1949; 61:203–208. 5 pl

32. Freedberg RS, Goodkin GM, Perez JL, Tunick PA, Kronzon I. Valve strands are strongly associated with systemic embolization: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995; 26:1709–1712.

33. Roldan CA, Shively BK, Crawford MH. Valve excrescences: prevalence, evolution and risk for cardioembolism. J Am Coll Cardiol. 1997; 30:1308–1314.

34. Aziz F, Baciewicz FA Jr. Lambl's excrescences: review and recommendations. Tex Heart Inst J. 2007; 34:366–368.
35. Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol. 1997; 30:784–790.

36. Ngaage DL, Mullany CJ, Daly RC, Dearani JA, Edwards WD, Tazelaar HD, McGregor CG, Orszulak TA, Puga FJ, Schaff HV, Sundt TM 3rd, Zehr KJ. Surgical treatment of cardiac papillary fibroelastoma: a single center experience with eighty-eight patients. Ann Thorac Surg. 2005; 80:1712–1718.

37. Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003; 146:404–410.

38. Lammers RJ, Bloor CM. Cancer and the heart. New York: Springer-Verlag;1986.
39. Melduni RM, Klarich KW, Nesbitt GC, Shub C. Lambl's excrescences: is surgical excision really necessary? Tex Heart Inst J. 2008; 35:89. author reply 90.
40. D'Avila A, Scanavacca M, Sosa E, Ruskin JN, Reddy VY. Pericardial anatomy for the interventional electrophysiologist. J Cardiovasc Electrophysiol. 2003; 14:422–430.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download