Abstract
A subaortic membrane is an uncommon cause for left ventricular outflow tract obstruction. Hypertrophic cardiomyopathy with dynamic left ventricular outflow tract obstruction would mask the presence of the subaortic membrane on transthoracic echocardiography and cause a false diagnosis. We report a patient with subaortic stenosis due to flail subaortic membrane misdiagnosed as obstructive hypertrophic cardiomyopathy on transthoracic echocardiography, identified on transesophageal echocardiography and cardiac catheterization.
Subaortic membrane is an uncommon cause of the left ventricular outflow tract (LVOT) obstruction. It is important to distinguish a dynamic LVOT obstruction from fixed LVOT obstruction by a subaortic membrane. Transthoracic echocardiography (TTE) could miss the subaortic membrane close to the aortic valve; transesophageal echocardiography (TEE) could finely visualize subvalvular and supravalvular structures and help to find the other cause of LVOT obstruction such as subaortic membrane.
We report a case of patient who had a flail subaortic membrane with dynamic LVOT obstruction misdiagnosed as obstructive hypertrophic cardiomyopathy (HCMP) with dynamic LVOT obstruction; the subaortic membrane was not seen initially on TTE, but identified by TEE and cardiac catheterization.
A 67-year-old female presented to our hospital with a symptom of gradually aggravated dyspnea. Clinical examination confirmed the grade 3/6, subaortic, midsystolic murmur and increased respiration rate of 28 breaths/min. A 12-lead electrocardiography showed left ventricular hypertrophy in voltage criteria. A chest radiograph demonstrated marked cardiomegaly with pulmonary edema (Fig. 1).
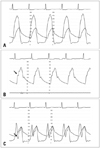
Eight years ago, the patient had come to our hospital with similar symptoms. On TTE, the LV interventricular septal wall thickness and LV posterior wall thickness were 15 mm and 10 mm at diastolic phase, respectively, and papillary muscle was hypertrophied. There was no significant calcification, thickening or motion limitation of aortic valve to increase flow velocity. Continuous wave (CW) Doppler spectrum did not show late peaking appearance but symmetrical appearance and the velocity was increased up to 6 m/sec at the LVOT level during the resting state. Therefore we had regarded the patient as having HCMP accompanied by flow acceleration caused by narrow LVOT (Fig. 2). In this time, TTE was of suboptimal quality but suggested the presence of hypertrophied interventricular septum and turbulent flow at the basal interventricular septum, which findings were similar to those by the previous TTE. The CW Doppler showed slightly late peaking configuration and the peak pressure gradient between the LV and the ascending aorta was 151 mmHg. However, there were no definite aortic stenosis and systolic anterior motion (SAM) of anterior mitral valve leaflet or chordae to induce the high pressure gradient between the LV and the ascending aorta. TEE was performed to find out the cause for the high pressure gradient between the LV and the ascending aorta; confirmed the flail subaortic membrane which disturbs the forward flow toward the ascending aorta and causes severe subaortic stenosis (Fig. 3). To identify the hemodynamic significance of the flail subaortic membrane, we performed cardiac catheterization. We simultaneously recorded left ventricular pressure and aortic pressure using right radial long sheath. There was a pressure drop at systolic phase on the pressure curve of the LVOT. The pressure drop coincided with the notch which was measured at systolic phase of ascending aorta pressure curve (Fig. 4). These pressure curve changes implied that the subaortic membrane of interventricular septum has a critical role in inducing high pressure gradient between the LVOT and the ascending aorta. She had an open heart surgery for the resection of subaortic membrane. After original planned resection of subaortic membrane, the operator thought that interventricular septal myectomy and mitral valvular replacement would be helpful. Because she had severe LV hypertrophy due to longstanding subaortic membrane, it looks like HCMP. Aortic valvuloplasty and papillary muscle release were done due to incidental papillary muscle rupture. Her symptoms were improved after the resection of subaortic membrane and she was discharged without major complications.
Subaortic membrane is a rare congenital heart disease and one of the pathologies of the ventricular hypertrophy in adults but never recognized in early infancy.1)2) It is thought that underlying genetic predisposition and various geometric and anatomical variations of LVOT leading to flow turbulence result in the subaortic membrane.3)
The echocardiographic assessment of the severity and the cause of LVOT obstruction is a very important in terms of its impact on the clinical outcome.4) Differential diagnosis between subaortic membrane and obstructive HCMP could be difficult. As subaortic membrane is infrequent cause of LVOT obstruction in adulthood, HCMP and dynamic LVOT obstruction would mask the presence of the subaortic membrane and cause a false diagnosis as obstructive HCMP.5)
Although most of subaortic stenosis is usually a fixed lesion such as fibrous ridge rather than mobile membrane,3)6) flail subaortic membrane diagnosed with TTE was also documented.7)
Unlike this report, we initially misdiagnosed the patient as having obstructive HCMP by TTE. Obstructive HCMP usually has the following characteristics: LVOT obstruction, SAM of the anterior leaflet of the mitral valve or chordae, and mitral regurgitation.8) However, our patient showed increased pressure gradient between LV and aorta but no definite SAM of anterior mitral valve leaflet or chordae and mitral regurgitation on TTE; detect subaortic membrane on TEE. Thus, it is clear that not all cases of LVOT obstruction are due to septal hypertrophy. TEE is more useful than TTE in visualizing perivalvular structures and would help to confirm the presence of unusual causes for severe LVOT obstruction and left ventricular hypertrophy, such as subvalvular or supravalvular stenosis; cardiac catheterization would aid to find the hemodynamic impact of the pathologic lesions.
In conclusion, meticulous evaluation including TEE and cardiac catheterization would be necessary to confirm various causes for the LVOT obstruction, especially undetected subaortic membrane on TTE.
Figures and Tables
Fig. 1
Precordial leads of electrocardiogram show left ventricular hypertrophy in voltage criteria rather than deep T wave showing in hypertrophic cardiomyopathy (A). Chest radiography shows marked cardiomegaly with pulmonary edema (B).
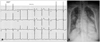
Fig. 2
Left ventricular (LV) interventricular septal wall thickness and LV posterior wall thickness were 15 mm and 10 mm on parasternal long axis view at diastolic phase and papillary muscle was hypertrophied (arrow) (A). Color Doppler of 2D echocardiography shows flow acceleration at the left ventricular outflow tract (LVOT) level of interventricular septum (B and C). Continuous wave Doppler spectrum was not late peaking appearance but symmetrical appearance and the velocity was increased up to 6 m/sec at the LVOT level during resting state (D).
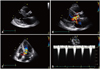
Fig. 3
One hundred and five degree color compared view of transesophageal echocardiography shows the linear mobile subaortic membrane on basal interventricular septum (arrow). The subaortic membrane disturbs the forward flow toward ascending aorta.
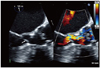
Fig. 4
(A) Double pressure tracing was performed using radial sheath and coronary catheter in left ventricle. (B) Aortic pressure curve was recorded at the ascending aorta level. There is notch on systolic phase of pressure curve (arrow). (C) Aortic pressure curve was recorded and left ventricle pressure curve was recorded at the left ventricular outflow tract (LVOT) level. There was pressure drop during systolic phase on pressure curve recorded at the LVOT level (arrow). The pressure drop was caused by dynamic motion of subaortic membrane during cardiac cycle. The pressure drop is consistent with the notch of ascending aorta pressure curve of Fig. 4B.
References
1. Firpo C, Maitre Azcárate MJ, Quero Jiménez M, Saravalli O. Discrete subaortic stenosis (DSS) in childhood: a congenital or acquired disease? Follow-up in 65 patients. Eur Heart J. 1990; 11:1033–1040.

2. Choi JY, Sullivan ID. Fixed subaortic stenosis: anatomical spectrum and nature of progression. Br Heart J. 1991; 65:280–286.

3. Teis A, Sheppard MN, Alpendurada F. Subaortic membrane: correlation of imaging with pathology. Eur Heart J. 2010; 31:2822.

4. Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003; 348:295–303.

5. Bruce CJ, Nishimura RA, Tajik AJ, Schaff HV, Danielson GK. Fixed left ventricular outflow tract obstruction in presumed hypertrophic obstructive cardiomyopathy: implications for therapy. Ann Thorac Surg. 1999; 68:100–104.

6. Oliver JM, González A, Gallego P, Sánchez-Recalde A, Benito F, Mesa JM. Discrete subaortic stenosis in adults: increased prevalence and slow rate of progression of the obstruction and aortic regurgitation. J Am Coll Cardiol. 2001; 38:835–842.

7. Onbasili AO, Tekten T, Ceyhan C. Subaortic stenosis caused by flail discrete membrane in an older patient. Heart. 2004; 90:399.

8. Nagueh SF, Bierig SM, Budoff MJ, Desai M, Dilsizian V, Eidem B, Goldstein SA, Hung J, Maron MS, Ommen SR, Woo A. American Society of Echocardiography. American Society of Nuclear Cardiology. Society for Cardiovascular Magnetic Resonance. Society of Cardiovascular Computed Tomography. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: Endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2011; 24:473–498.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download