Abstract
We present a rare case involving a ruptured sinus of Valsalva aneurysm (SVA) and acute myocardial infarction in a 39-year-old male patient. Coronary angiography showed normal findings; however, the patient showed remarkably elevated levels of cardiac enzymes and decreased left ventricular function with apical akinesia on transthoracic echocardiography. Transesophageal echocardiography revealed shunt flow from the SVA to the right atrium without significant aortic regurgitation. Preoperative cardiac arrest was managed by cardiopulmonary resuscitation, and surgical repair was performed by closing the entrance of the aneurysm. However, the compromised hemodynamic status was not reversed by surgery.
Sinus of Valsalva aneurysm (SVA) is a rare cardiac anomaly and is involved in less than 1% of cardiac operations.1) Most patients with unruptured SVAs are asymptomatic; however, a ruptured SVA results in a devastating course and causes various clinical manifestations such as aortic regurgitations or arrhythmic disorders. Acute coronary syndrome may occur with compression of the coronary artery2) or severe acute aortic regurgitation. We present a case of a ruptured SVA mimicking acute myocardial infarction (AMI), without either significant coronary artery compression or aortic regurgitation.
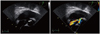
A 39-year-old male patient presented with chest discomfort and dyspnea that had lasted for 12 hours. A grade 3/6 diastolic murmur was auscultated at the left third intercostal space, and an electrocardiogram showed mild ST segment depression on precordial leads (Fig. 1). The creatine kinase MB (CK-MB), troponin T, and serum creatinine levels were 208.4 ng/mL (reference value, < 5 ng/mL), 2.54 ng/mL (reference value, < 0.1 ng/mL), and 4.1 mg/dL, respectively. Transthoracic echocardiography (TTE) showed slightly reduced left ventricular systolic function (ejection fraction, 51%) with apical akinesia and mild aortic regurgitation (grade 1). The pattern of apical akinesia was dissimilar to that associated with the apical ballooning caused by stress-induced cardiomyopathy. The patient had unstable vital signs, and his echo window was very poor due to severe obesity; a thorough echocardiographic study was impossible. Emergency coronary angiography was planned under the impression of AMI, but cardiac arrest occurred immediately before coronary angiography. Therefore, cardiopulmonary resuscitation (CPR) was performed. Coronary angiography revealed no significant luminal narrowing. Coronary flow was within normal limits, and there was no evidence of plaque disruption or thrombosis. However, an aortogram showed a shunt from the aorta to the right heart. Cardiac catheterization showed elevated right ventricular and pulmonary artery pressure (peak, 55 mmHg). The Qp/Qs was estimated to be approximately 3.15. Subsequent transesophageal echocardiography (TEE) revealed shunted flow from the SVA of right coronary cusp to the right atrium and grade 1 aortic regurgitation (Fig. 2).
Emergency repair of the ruptured SVA was performed. Exposure of the ascending aorta and right atrium revealed a SVA on the right coronary cusp, which had ruptured to the right atrium (Fig. 3). The aortic valve was morphologically normal in appearance with no detectable valve dysfunction. After successful direct closure of the rupture site, intraoperative TEE result did not showed shunted flow or aortic valve dysfunction.
Cardiac function and mental status were not restored after surgery. Preoperative multiple organ failure (MOF) was aggravated despite intraaortic balloon pump with a high dose of cardiotonics and continuous renal replacement therapy. Electroencephalography showed that severe cerebral injury might have been caused by the prolonged preoperative CPR. The patient died on the ninth day after the operation.
The mechanism underlying AMI in ruptured SVAs has not been well identified. Most cases of AMI, with SVAs, are associated with a left sinus origin and compression of the left coronary artery.3) Another hypothesis is that aortic regurgitation and a left-to-right shunt lead to a severe coronary oxygen supply-demand mismatch, causing myocardial ischemia.4)5) In this case, SVA originated from the right sinus, and there was neither significant aortic regurgitation nor compression of the coronary artery.
The initial TTE study did not yield a correct diagnosis because of a poor echo window and the patient's lack of cooperation in facilitating a thorough examination. Even with the indefinite findings of the electrocardiogram, primary coronary angiography was mandatory owing to the regional wall motion abnormality and the markedly elevated cardiac enzyme levels. The possible mechanisms of myocardial infarction, without coronary compression or severe aortic regurgitation, are as follows: acute, severe shunt from the aorta to the right heart causing systemic hypoxia and flow insufficiency to the coronary artery, and decompensation, with left ventricular dysfunction, caused by myocardial infarction-aggravated systemic and myocardial ischemia.
Although unruptured SVAs are mostly asymptomatic, fatal complications such as right ventricular outflow obstruction, malignant arrhythmias, and acute coronary syndrome can occur in ruptured SVAs. AMI, with a ruptured SVA, is a rare condition but can occur. Acute, massive left-to-right shunts can cause myocardial infarction and rapidly progressive MOF, without definite obstruction of coronary blood flow or severe aortic regurgitation. With the experience of this case, the possibility of SVA rupture should be considered in cases of AMI without significant coronary obstruction; careful echocardiographic evaluation is needed.
Figures and Tables
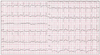
Fig. 1
An electrocardiogram obtained on admission showing sinus tachycardia and subtle ST depression in V5-6.
References
1. Takach TJ, Reul GJ, Duncan JM, Cooley DA, Livesay JJ, Ott DA, Frazier OH. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999. 68:1573–1577.

2. Cuculi F, Rossi M, Bradley KM, Westaby S. Rupture of a left sinus of valsalva aneurysm with coronary compression: a rare cause of ischemic chest pain. Ann Thorac Surg. 2011. 92:e97–e99.

3. Regueiro Abel M, Penas Lado M, López Ciudad V, Castro Beiras A. [Sinus of Valsalva aneurysm as a cause of acute myocardial infarction]. Rev Esp Cardiol. 2002. 55:77–79.
4. van Son JA, Danielson GK, Schaff HV, Orszulak TA, Edwards WD, Seward JB. Long-term outcome of surgical repair of ruptured sinus of Valsalva aneurysm. Circulation. 1994. 90(5 Pt 2):II20–II29.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download