Abstract
Carbon monoxide is a nonirritant, odorless, colorless gas. Its effects are prominent in organs most sensitive to oxygen deprivation, such as the heart, brain, and kidney. Although less frequently, an association between thromboembolic events and carbon monoxide poisoning has been shown in the literatures. In this case, we report a case of atrial thrombus associated with carbon monoxide poisoning.
Carbon monoxide (CO) is a colorless, odorless, and nonirritant gas that is lighter than air, and it is a product of incomplete combustion of hydrocarbons.1) Accidental, suicidal or homicidal intoxications with CO have a long history. Acute CO poisoning is an important clinical problem and may lead large proportion of patients to fatal death. Moreover, frequent neurologic and cardiovascular consequences have been described.2-4) The neurologic manifestations of CO poisoning have been well described, and include headache, dizziness, weakness, nausea, and confusion.3)4) Cardiac consequences have been reported, including arrhythmias and electrocardiographic alterations, acute myocardial infarction, pulmonary edema, and cardiogenic shock.5)6) Among these, myocardial injury is common in patients with moderate to severe CO poisoning,2) manifested as elevated cardiac biomarkers and the changes of regional wall motion abnormality in echocardiography.7)
A 24-year-old female patient with no preexisting disease was brought to the emergency unit for altered mentality due to suicidal exposure to CO. The duration of the exposure was unclear. She showed a good general appearance, the level of consciousness was alert: Glasgow Coma Scale was measuring up to 15. Vital signs were blood pressure of 132/101 mmHg, pulse rate of 87/min, respiration rate of 20/min and body temperature of 35.4℃. Oxygen saturation measured using pulse oxymetry was 100% when 15 L/min oxygen was applied through a reservoir bag mask. She was 164 cm tall and weighed 50 kg, and her body mass index was 18.6 kg/m2. Lower and upper limb arterial and venous examination revealed normal circulation. In detailed system examinations, no pathological neurologic signs were detected.
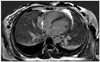
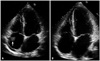
The chest radiography showed mild cardiomegaly with pulmonary edema in both lung fields. Laboratory analyses revealed the following: white blood cells, 21200/µL; blood urea nitrogen, 17 mg/dL; creatinine, 0.75 mg/dL, and blood glucose, 101 mg/dL, D-dimer 0.28 ug/mL. Cardiac enzymes were elevated (CK: 3306 U/L, CK-MB: 90.6 ng/mL, troponin I: 1.899 ng/mL, lactate dehydrogenase: 334 U/L). Arterial blood gas was performed and revealed pH 7.40, PaCO2 33 mmHg, PaO2 380 mmHg, HCO3 20 mmol/L, and the fraction of carboxyhemoglobin 16.0% (reference range < 2%). QT interval was prolonged in the electrocardiograph (ECG). The patient was transferred to the intensive care unit. At that time, she complained of newly onset chest pain, and cardiology consultation was requested. Pain was mainly retrosternal, lasted for several minutes, with no aggravating or relieving factors, and no change in position or respiration. ECG showed sinus rhythm with T wave inversion in precordial leads. Coronary computed tomography (CT) angiography performed to rule out coronary artery disease revealed normal coronary artery. Transthoracic echocardiography on the same day showed moderately reduced ejection fraction (42%), and akinesia of left ventricular apex. Stress induced cardiomyopathy or ischemic insult of left anterior descending artery were suspected. During comprehensive echocardiographic examination, echogenic mass with multiple nodularity in right atrium (RA) was identified. The size measured about 30 × 15 mm, and it appeared to be attached to the junction of superior vena cava. Transesophageal echocardiography and cardiac magnetic resonance imaging (MRI) were requested for further characterization of the mass. In mid-esophageal 140 degree view, a large nodular mass was attached to appendage of RA. Moreover, the mass was highly mobile and oscillating up and down across the tricuspid valve throughout the cardiac cycle (Fig. 1). The foremost diagnosis to exclude in this patient was intracardiac thrombus, and therefore we started anticoagulation therapy immediately. Cardiac MRI 1 day after anticoagulation therapy showed delayed enhancement suggestive of a thrombus, and an obvious reduction in size of the thrombus to 8 mm (Fig. 2). For the purpose of determining the etiology of thrombosis, she was questioned concerning recent potential precipitating conditions, such as surgery, immobilization and use of medications including oral contraceptives. Furthermore, she was evaluated for genetic and connective tissue disease leading to thrombophilic conditions. There were no identifiable risk factors for hypercoagulable state. Complete lower extremity ultrasound and abdominopelvic CT was conducted for suspicion of peripheral vein thrombosis and it demonstrated no thrombosis in other organs. The follow-up transthoracic echocardiogram was done on sixth day after anticoagulation, and showed no residual thrombus in the RA and normalized left ventricular (LV) systolic function (Fig. 3). The patient was discharged without any complication. The patient has been free of symptoms and there have been no clinical features of neurologic or thromboembolic complications during the 3-months of follow-up.
CO poisoning has special impact on organs sensitive to oxygen deprivation such as the heart, brain, and kidney. Myocardial injury assessed by ECG and cardiac enzyme elevation from moderate to severe CO poisoning is common (~40%) than expected.2) The proposed mechanism of global left ventricular dysfunction is tissue hypoxia and resultant myocardial stunning. The affinity of hemoglobin for CO is more than 200 times greater than its affinity for oxygen, and competitive inhibition of oxygen release leads to tissue hypoxia.3) Usually, the left ventricular dysfunction was transient and would be normalized with conventional treatment including high concentration of oxygen. In our case, left ventricular systolic dysfunction with regional wall motion abnormalities was associated with CO poisoning and recovered with conventional therapy.
In contrast, an association between thromboembolic accidents and CO poisoning has been shown less commonly in the literatures. To our knowledge, this is the first Korean case of acute CO poisoning combined with RA thrombus formation. Besides LV thrombus formation associated with transient apical ballooning of LV,15) there have been several reports regarding arterial and venous system thrombosis and related embolism including popliteal artery,8)9) superior sagittal sinus,10) vein of Labbe,11) mesenteric artery,12) basillar trunk,13) and popliteal vein.14) A plausible explanation for thromboembolic events is the effect of CO on platelets aggregation. CO poisoning leads to some changes in blood vessel and CO and nitric oxide exchange on platelet. Disturbed mitochondrial mechanisms by NO and its derivatives facilitate production of free oxygen radicals.16) Oxidative stress enhanced by free oxygen radicals may lead to endothelial damage, and subsequent platelet aggregation.17) Although the optimal therapy and duration of anticoagulation for CO induced RA thrombus is still unknown, the use of anticoagulant therapy in the acute phase and until complete resolution of thrombus appears to be appropriate in patients with atrial thrombus.18)19)
In conclusion, myocardial injury is common in CO poisoning. Thromboembolism could occur in cardiac chambers and peripheral vessels, although it was less frequent. Therefore, patients presented with CO poisoning should undergo echocardiographic examination followed by serial ECG and cardiac enzyme evaluation. Careful examination should be performed for the assessment of intracardiac thrombus, in addition to the exact evaluation of ventricle function.
Figures and Tables
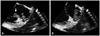
Fig. 1
Transesophageal echocardiography showed a mass (arrow) attached to right atrial appendage with to (A) and fro (B) motion through tricuspid valve.
References
1. Van Meter KW. Tintinalli JE, Kelen GD, Stapczynski JS, editors. American College of Emergency Physicians. Carbon monoxide poisoning. Emergency medicine: a comprehensive study guide. 2000. 5th ed. New York: McGraw-Hill;1303.
2. Satran D, Henry CR, Adkinson C, Nicholson CI, Bracha Y, Henry TD. Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. J Am Coll Cardiol. 2005. 45:1513–1516.

5. Marius-Nunez AL. Myocardial infarction with normal coronary arteries after acute exposure to carbon monoxide. Chest. 1990. 97:491–494.

6. Middleton GD, Ashby DW, Clark F. Delayed and longlasting electrocardiographic changes in carbon-monoxide poisoning. Lancet. 1961. 1:12–14.

7. Jang WI, Park JH. Transient left ventricular systolic dysfunction associated with carbon monoxide toxicity. J Cardiovasc Ultrasound. 2010. 18:12–15.

8. Enzer N, Spilberg S. Gangrene of lower extremity following carbon monoxide asphyxia. Am J Clin Pathol. 1946. 16:111–116.

10. Nagy Z, Kenéz J, Simon L, Mórocz K. [Partial thrombosis of the superior sagittal sinus following carbon monoxide poisoning]. Orv Hetil. 1984. 125:3181–3184.
11. Cambria S. [Thrombosis of the vein of Labbé with haemorrhagic cerebral infarction (author's transl)]. Rev Neurol (Paris). 1980. 136:321–326.
12. Condi M, Devaux C, Sallerin T. [A case of thrombosis of the mesenteric artery after carbon monoxide poisoning]. Anesth Analg (Paris). 1973. 30:353–358.
13. Breton J, Caroff J, Martin R, Dehouve A, Dehouve P. [Fatal obstruction of the basilar trunk following benign carbon monoxide poisoning]. Med Leg Dommage Corpor. 1969. 2:409–411.
14. Heidrich H, Klems H. [Bilateral thrombosis of the popliteal vein with diffuse muscular necrosis following CO intoxication]. Dtsch Med Wochenschr. 1969. 94:1367–1370. passim.

15. Lee SJ, Kang JH, Kim NY, Baek IW, Park MY, Shim BJ, Koh YS, Shin WS, Lee JM, Jeon HK. A case report of carbon monoxide poisoning induced cardiomyopathy complicated with left ventricular thrombus. J Cardiovasc Ultrasound. 2011. 19:83–86.

16. Davis MR, Fitzpatrick CM, Dixon PM, Kashyap VS. Thrombus-induced endothelial dysfunction: hemoglobin and fibrin decrease nitric oxide bioactivity without altering eNOS. J Surg Res. 2004. 122:121–129.

17. Felner JM, Churchwell AL, Murphy DA. Right atrial thromboemboli: clinical, echocardiographic and pathophysiologic manifestations. J Am Coll Cardiol. 1984. 4:1041–1051.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download