Abstract
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital anomaly associated with very high mortality during infancy. We report a 35-year-old female patient with ALCAPA initially visualized by echocardiography. She visited outpatient department presenting with intermittent chest discomfort for 3 weeks. Transthoracic echocardiography showed left coronary artery arising from main pulmonary artery and abundant septal color flow Doppler signals. Transesophageal echocardiography clearly revealed markedly dilated and tortuous right coronary artery showing windsock appearance. Multidetector computed tomography and coronary angiography enabled visualization of anomalous left coronary artery originating from left side of main pulmonary trunk. After treadmill exercise test which showed ST-segment depression presenting inducible myocardial ischemia, patient underwent direct re-implantation of the anomalous coronary artery into the aorta without any complication.
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare coronary anomaly comprising 0.2-0.5% of all congenital cardiac malformations. Although ALCAPA has high mortality of 90% within the 1st year of life, 10-15% of patients survive to adulthood in case of existence of extensive intercoronary collaterals.1) In the past, ALCAPA was diagnosed exclusively by cardiac catheterization and angiography.2) However, recent technical advances in echocardiographic imaging and multidetector computed tomography (MDCT) coronary angiography enabled better visualization of the anomalous origin of coronary artery and associated findings. In Korea, there have been several reports of adult type ALCAPA.3-5) Most of them are diagnosed by invasive coronary angiography with or without diagnostic clue of echocardiography. Here, we report a 35-year-old female patient with ALCAPA initially visualized by echocardiography and MDCT coronary angiography.
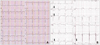
A 35-year-old female visited outpatient department presenting with intermittent chest discomfort for 3 weeks. She had been generally in healthy condition and had no known coronary risk factors. There was no family history of heart disease or sudden death. On physical examination, grade 3 systolic murmur was heard at left sternal border. There was no specific abnormality in plain chest radiography. Electrocardiography showed precordial T wave inversion thought to be juvenile pattern. Transthoracic echocardiography showed left coronary artery (LCA) arising from main pulmonary artery and abundant septal color flow Doppler signals (Fig. 1A, B and C). There was abnormal turbulent flow adjacent to the right atrium thought to be of giant tortuous right coronary artery (RCA). Transesophageal echocardiography clearly revealed markedly dilated and tortuous RCA showing windsock appearance (Fig. 1D). The ratio of the proximal RCA diameter to the aortic root diameter was markedly increased (RCA: aorta ratio = 0.217). Left ventricular function and regional wall motion was normal. MDCT coronary angiography showed LCA originating from the left side of the pulmonary artery and remarkably dilated and tortuous RCA consistent with the findings of echocardiography (Fig. 2A). In coronary angiography, we could not engage LCA. RCA was extraordinarily dilated and tortuous with abundant collateral channels draining into the pulmonary artery through LCA (Fig. 2B). Treadmill exercise test showed ST-segment depression in lead II, III, aVF and V6 presenting inducible myocardial ischemia (Fig. 3). Patient was undergone direct re-implantation of the anomalous coronary artery into the aorta and pericardial patch closure of main pulmonary artery without any complication (Fig. 4). Transient left ventricular hypertrophy was found for several days after the operation, which was supposed to be from increased blood flow to LCA from aorta. At present, six months after surgical correction of ALCAPA, patient is in good condition without any complication and cardiac symptom.
Positional anomalies of the coronary arteries are caused by abnormalities in embryologic development. The most common is ALCAPA, followed by anomalous RCA from the pulmonary artery (ARCAPA).6) Most of patients die within the 1st year of life. Even though 10-15% of patients survive to adulthood, they are prone to sudden death secondary to malignant ventricular arrhythmias, myocardial ischemia or global cardiomyopathy with an estimated incidence of 80-90% at a mean age of 35 years. After first description of ALCAPA by Brooks7) in 1885, small number of ALCAPA cases were reported from place to place. In Korea, O et al.3) reported 45-year-old man with ALCAPA diagnosed by invasive coronary angiography without any clue from transthoracic echocardiography in 1993.
Clinical features of adult case of ALCAPA are somewhat different from those of infancy. Infants with ALCAPA usually suffer from symptoms of heart failure or myocardial ischemia/infarction manifested as perioral cyanosis, shortness of breath, diaphoresis. In adult patients with ALCAPA, clinical manifestations are variable from asymptomatic murmur to syncope or sudden death. Non specific systolic murmur can be heard commonly on physical examination and ischemic T wave changes are found in electrocardiography in most cases. The clinical diagnosis of ALCAPA is not easy because this lesion is rare and clinical findings are frequently indistinguishable from those of other cardiac pathology. Up to recently, definite diagnosis of ALCAPA has been made by conventional coronary angiography.8) However recent advances of imaging technique such as echocardiography, MDCT, and magnetic resonance imaging enabled noninvasive morphological assessment of the coronary arteries. Rha et al.5) reported a 65-year-old woman with ALCAPA confirmed by coronary angiography with a clue of dilated RCA in transthoracic echocardiography. After conventional coronary angiography, they also performed MDCT coronary angiography for three dimensional visualization of coronary anomaly. Echocardiographic findings of ALCAPA include dilated RCA and detection of pulmonary artery to LCA shunt with abundant septal collaterals by both pulse wave and color flow Doppler imaging.2) In our case, detection of above echocardiographic findings was not so difficult. Subsequent MDCT coronary angiography visualized three dimensional coronary anatomy consistent with echocardiographic findings, which was verified by conventional coronary angiography.
Because patients with ALCAPA have potential risk of sudden death due to malignant arrhythmias or myocardial ischemia, surgical intervention is the definitive treatment for ALCAPA.9) Sometimes, adult patient with ALCAPA is often clinically asymptomatic or presents with only atypical chest discomfort without obvious abnormality in routine screening test. The detection of unusual color flow signals should raise one's suspicion coronary artery abnormality. Our case gives us emphasis on the need of close attention during echocardiographic study including color flow Doppler exam of ventricular septum and identification of origin of coronary arteries.
Figures and Tables
Fig. 1
Transthoracic echocardiography showed LCA arising from main PA (A) and abundant septal color flow signals in apical four chamber view (B) and pulse wave Doppler signals in parasternal short axis view (C). Transesophageal echocardiography revealed markedly dilated and tortuous RCA showing windsock appearance (D). AO: aorta, PA: pulmonary artery, PV: pulmonic valve, LCA OS: left coronary artery ostium, RA: right atrium, LA: left atrium, RCA: right coronary artery.
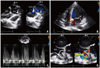
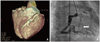
Fig. 2
A: Three dimensional volume rendering image of multidetector computed tomography coronary angiography showed LCA beginning from left side of the PA. B: In coronary angiography, right coronary artery was extraordinarily dilated and tortuous with abundant collateral channels (arrow) draining into PA through LCA. LCA: left coronary artery, PA: pulmonary artery.
References
1. Murala JS, Sankar MN, Agarwal R, Golla PN, Nayar PG, Cherian KM. Anomalous origin of left coronary artery from pulmonary artery in adults. Asian Cardiovasc Thorac Ann. 2006. 14:38–42.

2. Frommelt MA, Miller E, Williamson J, Bergstrom S. Detection of septal coronary collaterals by color flow Doppler mapping is a marker for anomalous origin of a coronary artery from the pulmonary artery. J Am Soc Echocardiogr. 2002. 15:259–263.

3. O SI, Lim HJ, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW, Kim JH. A case of anomalous left coronary artery from pulmonary artery (Bland-White-garland sysndrome). Korean Circ J. 1993. 23:468–473.

4. Shon TS, Moon KW, Yoo KD, Youn HJ, So SC, Kwak KK, Park HK, Chung WS, Han SK, Hong SJ. Anomalous origin of left coronary artery from pulmonary artery: report of an adult case. Korean Circ J. 1999. 29:528–531.

5. Rha SW, Park CG, Yong HS, Suh SY, Moon SK, Hong SJ, Kim JW, Seo HS, Oh DJ, Ro YM. Anomalous origin of the left coronary artery from the pulmonary artery in an elderly patient visualized by three-dimensional multidetector computed tomograph coronary angiography. Korean Circ J. 2005. 35:84–87.

6. Williams IA, Gersony WM, Hellenbrand WE. Anomalous right coronary artery arising from the pulmonary artery: a report of 7 cases and a review of the literature. Am Heart J. 2006. 152:1004.e9–1004.e17.

7. Brooks HS. Two cases of an abnormal coronary artery of the heart arising from the pulmonary artery: with some remarks upon the effect of this anomaly in producing cirsoid dilatation of the vessels. J Anat Physiol. 1885. 20(Pt 1):26–29.
8. Fisher EA, Sepehri B, Lendrum B, Luken J, Levitsky S. Two-dimensional echocardiographic visualization of the left coronary artery in anomalous origin of the left coronary artery from the pulmonary artery. Pre- and postoperative studies. Circulation. 1981. 63:698–704.

9. Kim KW, Park KY, Choi CH, Park CH, Jeon YB, Lee JI. Anomalous origin of the left coronary artery from the pulmonary artery in an adult: a case report. Korean J Thorac Cardiovasc Surg. 2007. 40:503–507.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download