Introduction
Although the primary tumors of the heart are extremely uncommon (various post mortem studies report rates between 0.001% and 0.28%), the heart is not a rare site of metastasis of various malignant tumors.1-4) Lung cancer is one of the most common primary tumor of cardiac metastasis, and adenocarcinoma is the most frequent cell type.2)4) Cardiac metastasis of lung cancer usually involve the pericardium or epicardium by directly invasion and/or lymphatic spread, however metastasis to the left ventricular (LV) myocardium and endocardium is extremely rare.2)3)5) We report a case of patient with adenocarcinoma of lung, metastasized to the LV myocardium and endocardium, which diagnosed by echocardiography and 18-fludeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT).
Case
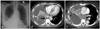
A 53-year-old woman was admitted to our hospital due to right pleuritic chest pain, accompanied with hemoptysis, fever and chilling sensation for 3 days. Her blood pressure was 120/80 mmHg, pulse rate was 87 beats per minute and body temperature was 37.1℃. On physical examination, crackle was heard in the right lower lung field and she complained tenderness in right chest wall. Her heart beat was regular and murmur was not auscultated. The electrocardiogram showed normal sinus rhythm with heart rate 79 beats per minute. On laboratory examination, cardiac enzymes were normal, white blood cell count was slightly elevated (13530/mm3), erythrocyte sedimentation rate (22 mm/hr) and C-reactive protein (3.62 mg/L) were within normal range. Plain chest X-ray showed soft tissue fullness at right infra-hilar area and air-fluid level in right lower lung field (Fig. 1A). Chest CT revealed cavitary lung mass in the right lower lobe and multiple lymphadenopathies in right side mediastinum (Fig. 1B and C). Bronchoscopy revealed multiple nodules at right intermediate bronchus and right second carina. Biopsy was performed at right intermediate bronchus and histological diagnosis of biopsy specimen revealed poorly differentiated adenocarcinoma. Brain magnetic resonance imaging (MRI) was performed for staging of lung cancer. On the brain MRI, there was no evidence of metastatic lesion. However on diffusion weighted image, high signal intensity spots, which show low signal intensity at apparent diffusion coefficient map were noted in both parietal cortex. This finding suggested acute embolic infarction.
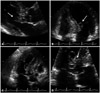
To evaluate the cardiac origin of embolic infarction, transthoracic echocardiography (TTE) was performed. TTE showed normal sized cardiac chambers with normal LV systolic function, and there were no pericardial effusion or outside compressing mass. However 2.4 × 1.4 cm sized hyper-mobile, multi-lobulated, cystic mass was observed at the LV outflow track (LVOT). The mass had connection with the basal portion of the interventricular septum (IVS) by narrow stalk. Although the mass was protruded to the LV lumen, there was no significant flow obstruction in LVOT (Fig. 2).
To differentiate the character of LVOT mass, PET-CT was performed. On PET-CT, hyper-metabolic mass [maximal standardized uptake value (SUVmax) = 14.8] with central necrosis was observed at right lower lobe and multiple FDG uptaking lymph-nodes were observed in the right hilum and subcarinal mediastinum. Intense FDG uptaking mass (2.5 × 2.3 cm) was also noted at IVS of LV (SUVmax = 13.9) suggesting metastasis of lung cancer (Fig. 3).
She didn't have any cardiac symptoms related to LV mass and there was no neurologic symptom associated with embolic infarction, and her lung cancer stage was IV, we made decision not to operate her cardiac lesion. She received one cycle of chemotherapy for metastatic lung adenocarcinoma and discharged. However, she couldn't receive another cycle of chemotherapy due to poor general condition, and expired due to respiratory failure after two months.
Discussion
The heart is frequently the site of metastasis of various malignant tumors and metastatic tumors are 20 to 40 times more common than primary tumors.6) The most common neoplasm associated with cardiac metastasis are lung cancer, lymphoma, breast cancer, leukemia, stomach cancer and melanoma.1)4)6) In autopsy series, cardiac metastasis may be present in up to 15% to 35% of patients with lung cancer.1)4)6-8) In case of cardiac metastasis, pericardium is most commonly affected structure and the involvement is either the result of direct invasion or lymphatic spread. Epicardium and myocardium is the next frequent site of metastasis and almost exclusively the result of retrograde lymphatic spread.1)4)8) However, lung cancer involving endocardium is extremely rare and usually the result of the hematogenous seeding from the bloodstream of the heart's chambers with intracavitary lodging or secondary to diffusion from myocardial metastasis. The literatures reporting on endocardial metastasis of the lung cancer are very scarce. Kasai et al.9) reported the case of lung adenocarcinoma developed a large mass in the ventricular septum, complete atrioventricular block, and obstruction of the left ventricular outflow tract. Che et al.5) reported the case of primary lung carcinoma metastasis to the heart accompanied by an intracavitary pedunculated mass in the LV. Bussani et al.4) reported that, there were no cases of metastasis spreading to the endocardium among the 96 cases of lung adenocarcinoma. Moreover, in case of metastasis involving endocardium, the metastatic lesions involving left ventricle as our patient are very rare and are mainly located in the right ventricle or atrium, because anchorage of cancer cells is favored by the low intracavitary pressure, slower blood flow, and the lighter contractile strength of the right heart chambers.4)
Although echocardiography has definite advantages for evaluating cardiac masses, it is difficult to distinguish malignant metastatic mass from other cardiac masses such as primary cardiac tumor, myxoma, vegetation, or thrombus by echocardiography alone. Comprehensive evaluation of cardiac mass, including use of other imaging modalities (such as CT or MRI) and assessment of the patient's clinical history and other laboratory finding, may be needed for optimal clinical decision making. Especially, the cardiac MRI is very useful imaging modality for soft-tissue characterization and frequently used for localizing and analyzing the morphological appearance and infiltration of cardiac and juxta-cardiac structures.10) However, our patient performed the FDG PET-CT instead of cardiac MRI, because PET-CT not only has advantage in the differentiation between benign and malignant lesions of the heart but also can be useful for staging of the lung cancer by detecting the metastatic lesions in other sites, which may be difficult with other imaging modalities.
In our case, multi-lobulated, cystic mass was located at the endocardium of LVOT and had connection with myocardium of IVS. This mass was clinically considered as lung cancer metastasis to heart, however, other conditions, such as tumor thrombus or vegetation must be considered as differential diagnosis. The morphologic feature of cystic component within the mass is very unusual finding for thrombus and FDG uptake in PET-CT is also not compatible with thrombus. Infectious lesions in infective endocarditis also can demonstrate FDG uptake in PET-CT.11) However, as Bryant and Cerfolio12) reported, average SUVmax value of adenocarcinoma of lung (9.4) is typically higher than that of infection (5.1). The SUVmax of LVOT mass in our patient was 13.9, which was more compatible with malignancy. Also there were no clinical symptom or sign and laboratory features suggesting infective endocarditis.
Possible pathogenesis for the endocardial metastasis of lung cancer can be either 1) hematogenous seeding or 2) diffusion from myocardial metastasis. In the case of myocardial metastasis, lymphatic channel and pericardial effusion are common.8) Although the exact metastatic mechanism can't be clearly determined, because there was no pericardial effusion in our case, the former route is more likely to be the pathogenesis.
This is, as far as we know, the first case report of primary lung adenocarcinoma metastasized to the LV endocardium diagnosed by echocardiography and FDG PET-CT scan in Korea. Definite diagnosis of LV mass can only be made by surgical resection. However, when taking into consideration that 1) the patient had primary lung cancer, 2) primary malignant cardiac tumor is extremely rare, 3) the echocardiographic and clinical findings of this patient were not compatible with infective endocarditis or thrombus, and 4) intense FDG uptake at IVS of LV, we could make clinical decision that LV mass lesion is lung cancer metastasis to heart.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download