Abstract
A hypertrophied muscle band (HMB) in the left ventricle (LV), which can be misinterpreted as apical hypertrophic cardiomyopathy, is a rare echocardiographic finding in a patient with normal LV wall thickness. Not only are symptoms produced, but changes in the electrocardiogram (ECG) are limited to the repolarization phase and show no progression even in a large HMB. Hence, we report a case of a 25-year-old woman who visited a local medical clinic due to epigastric discomfort in January 2007. The 24-hour Holter ECG showed multiple premature ventricular complexes. An HMB (3.23 × 10.8 cm) was observed on two-dimensional echocardiography that ran toward the interventricular septum (IVS) across the LV and divided the LV into apical and basal cavities at the apical one-third of the LV. Although LV wall thickness showed normal range, flow acceleration was observed between the HMB and IVS and revealed dagger-shaped with a high pressure gradient up to 30 mmHg in continuous wave Doppler examination. Circumferential band-like myocardial hypertrophy was observed at the LV apex on cardiac magnetic resonance imaging. Myocardial thinning and prominent trabeculae were present from the proximal to distal HMB. However, contractility was normal at the myocardial thinning site, regional wall motion abnormality was not observed in cine images. Focal fatty accumulation was evident at the base of the HMB. Coronary angiography revealed no significant stenosis, whereas left ventriculography showed septation at the apical one-third of the LV. The patient was discharged without any medication.
A hypertrophied muscle band (HMB) in the left ventricle (LV), which can be misinterpreted as apical hypertrophic cardiomyopathy (AHC), is a rare echocardiographic finding in patients with normal LV wall thickness.1) Not only are symptoms produced, but changes in electrocardiograms (ECG) are limited to the repolarization phase and show no progression even in a large HMB.2) We report the case of a woman with changes in ECG in whom the final diagnosis was an HMB within the LV cavity after a complete clinical examination, echocardiography, and coronary angiography. Although this disease can be found in all age groups, including adults and children in western countries,1)2) no such reports have been published in Korea.
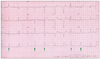
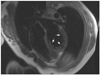
We report the case of a 25-year-old woman who had an HMB in the LV. She visited Kyungpook National University Hospital due to intermittent palpitations and vague chest discomfort. She was a non-alcoholic, had no cardiovascular risk factors, and her family history was unremarkable. Her initial vital signs were stable, and other physical examinations showed non-specific findings except for the irregular heart beat. Her ECG showed an intermittent junctional rhythm (Fig. 1), and the 24-hour Holter ECG revealed a sinus rhythm with intermittent junctional beats (1.9% of all cardiac beats). On 2-dimensional echocardiography, an HMB (3.23 × 10.8 cm) was observed that ran toward the interventricular septum (IVS), across the LV, and divided the LV into apical and basal cavities, which were at the apical one-third of the LV (Fig. 2). Although LV wall thickness showed normal range, flow acceleration was observed between the HMB and IVS and revealed dagger-shaped with a high pressure gradient up to 30 mmHg in continuous wave Doppler examination (Fig. 3). However, LV wall thickness was within the normal range, and apical contraction was good. Circumferential band-like myocardial hypertrophy was observed at the LV apex on cardiac magnetic resonance imaging (Fig. 4). Myocardial thinning and prominent trabeculae were evident from proximal to distal on the HMB. However, contractility was normal at the myocardial thinning site, and regional wall motion abnormality was not observed on a cine image. Focal fatty accumulation occurred at the base of the HMB. Coronary angiography showed no significant stenosis, whereas left ventriculography showed a septation in the apical one-third of the LV (Fig. 5). She was discharged without any medication.
A large HMB close to the LV apex can be misinterpreted as apical hypertrophy and may be diagnosed as AHC. Numerous patients with AHC experience a benign clinical course,3) whereas others suffer from angina and palpitations and may even develop a myocardial infarction with normal coronary arteries due to limited apical coronary flow reserve.2) However, no symptoms are produced by a large HMB.1) In our case, there was no symptom except mild palpitations and vague epigastric discomfort.
The ECG in cases of AHC shows giant negative T waves in the precordial leads, R waves of > 26 mm in lead V5, and an absence of septal Q waves, and these changes progress with time.4)5) In cases of HMB within the LV, the changes in ECG are limited to the repolarization phase and show no progression.2) Giant negative T waves can be obtained by assuming diffusely distributed hypertrophic cells at the apex.6) This is supported by the observation that a patient with AHC shows loss of giant T wave negativity after an apical myocardial infarction with development of a discrete apical aneurysm.7) In the case of a large HMB within the LV, the changes in ECG are probably produced by repolarization of this muscle trunk.2) In our case, no giant T wave negativity was evident despite the presence of some premature ventricular complexes.
Two-dimensional echocardiography in cases of AHC shows asymmetric hypertrophy confined predominantly to the LV apex, with an apical thickness of > 15 mm and maximal apical/posterobasal wall thickness ratio of > 1.5 : 1.0.7) In cases of HMB, both the septum and the LV apex have normal thicknesses.1) In our case, LV wall thickness was normal from the septum to the LV apex.
End-diastolic left ventriculography in the right anterior oblique projection in cases of AHC shows a spade-like configuration due to the diffusely circumferential hypertrophy at the apical level.5) LV injection in cases of a large HMB is normal.1) In our case, LV injection was normal on left ventriculography.
In addition, a double-chambered LV is a rare congenital anomaly, and only a few cases in which a two-chambered LV is separated by the IVS muscle bundle have been reported.8-10) This pathology is rarer than a double-chambered right ventricle.8)9)
In conclusion, although HMB and AHC can be misinterpreted as each other, the differential diagnosis between the two diseases is important, because the symptoms are present or may appear during follow-up, the changes in ECG are progressive, and the disease may be genetically transmitted in patients with AHC.
Figures and Tables
Fig. 2
Modified parasternal long-axis view showing the hypertrophied muscle band (arrow) that run toward the interventricular septum across the left ventricle (LV) and divide the LV into apical and basal cavities at its apical one-third region (A). Apical four-chamber view showing the hypertrophied muscle band (arrow) that run toward the interventricular septum, across the LV, and divide the LV into apical and basal cavities (B).
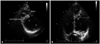
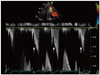
Fig. 3
Continuous wave Doppler examination showing flow acceleration between the hypertrophied muscle band and interventricular septum and dagger-shaped with a high pressure gradient up to 30 mmHg (arrows).
References
1. Salazar J. Left ventricular anomalous muscle band and electrocardiographic repolarization changes. Pediatr Cardiol. 1997. 18:434–436.

2. Sutton MG, Dubrey S, Oldershaw PJ. Muscular false tendons, aberrant left ventricular papillary musculature, and severe electrocardiographic repolarisation abnormalities: a new syndrome. Br Heart J. 1994. 71:187–190.

3. Maron BJ. Apical hypertrophic cardiomyopathy: the continuing saga. J Am Coll Cardiol. 1990. 15:91–93.

4. Sakamoto T, Tei C, Murayama M, Ichiyasu H, Hada Y. Giant T wave inversion as a manifestation of asymmetrical apical hypertrophy (AAH) of the left ventricle. Echocardiographic and ultrasono-cardiotomographic study. Jpn Heart J. 1976. 17:611–629.

5. Yamaguchi H, Ishimura T, Nishiyama S, Nagasaki F, Nakanishi S, Takatsu F, Nishijo T, Umeda T, Machii K. Hypertrophic nonobstructive cardiomyopathy with giant negative T waves (apical hypertrophy): ventriculographic and echocardiographic features in 30 patients. Am J Cardiol. 1979. 44:401–412.

6. Tsunakawa H, Wei D, Mashima S, Harumi K. Study on the genesis of giant negative T wave in apical hypertrophic cardiomyopathy using a three-dimensional computer model. Jpn Heart J. 1991. 32:799–809.

7. Webb JG, Sasson Z, Rakowski H, Liu P, Wigle ED. Apical hypertrophic cardiomyopathy: clinical follow-up and diagnostic correlates. J Am Coll Cardiol. 1990. 15:83–90.

8. Kay PH, Rigby M, Mulholland HC. Congenital double chambered left ventricle treated by exclusion of accessory chamber. Br Heart J. 1983. 49:195–198.

9. Vaidiyanathan D, Prabhakar D, Selvam K, Alagesan R, Thirunavukarasu N, Muthukumar D. Isolated congenital left ventricular diverticulum in adults. Indian Heart J. 2001. 53:211–213.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download