Introduction
Conventional two-dimensional (2D) transthoracic echocardiography has been greatly contributing in the clinical diagnosis of mitral regurgitation (MR) and it is certainly the clinical standard for the evaluation of valve apparatus. Nevertheless, 2D has a disadvantage in the assessment of three-dimensional (3D) morphology. Three-dimensional approach allows us to visually understand the valve configuration and gives us additional information that has never been provided by 2D approach. Another important aspect about 3D approach is that the reconstructed 3D images can be quantitatively measured and analyzed in 3D spaces. Three-dimensional transesophageal echocardiography (TEE), which has been widely available recently, can have a significant impact in pre-, post- and intraoperative assessment of mitral valve apparatus. These information play a critical role in decision making of the surgical strategy in organic or functional MR.
Mitral Valve Complex
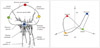
The whole mitral valve complex is consisted of annulus, leaflets, chordae tendineae, papillary muscles (PMs) and surrounding left ventricle (Fig. 1).1)2) With using multi-scanned 2D TEE images, well-trained echocardiographers can mentally reconstruct the 3D shape and position of the structures but it is not so easy to recognize or quantify the morphology and anatomical position of mitral valve complex. Some special software enable us to reconstruct the 3D image. The reconstructed 3D images can be quantitatively measured and analyzed in 3D spaces, which is impossible to do in 2D images. Three-dimensional evaluation of the mitral valve provides us important additional information in the clinical diagnosis, planning a surgical strategy or postoperative assessment.
Functional/Ischemic Mitral Regurgitation
Functional MR is defined as a regurgitation that is seen with structurally normal valve leaflets. Functional MR results from left ventricular remodeling such as dilatation or distortion, which displaces the PMs and then tethers the mitral leaflets, restricting leaflet coaptation.3)4) Ischemic MR is a complication of the chronic phase of myocardial infarction. It occurs commonly after myocardial infarction, with an estimated prevalence of 20% to 50%,5)6) and its presence has been shown to significantly worsen prognosis independent of ejection fraction.6)7) However, long-term effectiveness of the valve and ventricular repair surgery for the functional MR is still controversial.
Geometry in Functional/Ischemic MR
In a mitral valve with functional MR, geometric abnormalities of incomplete valve coaptation at systole are mainly characterized by leaflet tethering due to displacement of PMs and flattening of mitral annulus. These are caused by left ventricular remodeling, so that functional MR is called as ventricular disease rather than valvular disease. In patients with dilated cardiomyopathy, global ventricular remodeling occurs with resultant displacement of both PMs. In contrast, in patients with functional/ischemic MR due to inferior infarction, localized ventricular remodeling is more common with resultant posteromedial PM displacement.8) Real-time 3D echocardiography has proven that in patients with functional MR due to dilated cardiomyopathy, symmetrical PM displacement lead to progressive cordal tethering and leaflet tenting, resulting in predominantly central MR as a result of decreased leaflet coaptation.9-11) On the other hand, in patients with ischemic MR, left ventricular remodeling caused by abnormal wall motion results in uneven papillary displacement and asymmetric tethering associated with eccentric MR.9)12-14) Three dimensional TEE allows us to directly visualize the decreased leaflet coaptation and the differences of the coaptation depth in each segment.15)
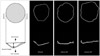
Mitral annulus shows a nonplanar saddle-shaped configuration in healthy individual.16) There are two peaks at the aortic insertion and posterior left ventricular wall and two low troughs closest to the apex located medially and laterally (Fig. 1).1)2)17) The saddle shape is most remarkable during mid-systole.18) The annulus acquires a more flattened shape at early-systole and end-diastole.19) The curvature contributes to the mechanism that mitral leaflets have an effective coaptation and to reduction the stresses imposed by left ventricular pressure during systole.20) Kwan et al.21) demonstrated the enlargement and less nonplanarity mainly in the anteroposterior direction during systole in patients with global left ventricular systolic dysfunction. These geometric changes were proportional to the global left ventricular systolic function. Our group has demonstrated dilatation and flattening of the mitral annulus in patients with ischemic MR22) and more deformation in anterior infarction compared to posterior infarction (Fig. 2 and 3).22)23)
Surgical Therapeutic Strategy for Functional MR
As previously mentioned, functional MR is a predictor of mortality in patient with left ventricular dysfunction. The survival of patients with functional MR, even with mild MR is significantly worse than in patients with the same degree of organic MR. It has been widely accepted that patients with moderate ischemic MR undergoing coronary artery bypass grafting should have concomitant mitral valve repair.24) However, the indication for surgical correction of mild to moderate functional MR is still a controversial issue. As a surgical procedure, mitral valve repair or mitral valve replacement is also controversial.25-29)
Need for Submitral Approach
Undersized annuloplasty has been widely accepted as a simple and at the same tine effective procedure.30)31) However it sometimes fails to ameliorate MR in patient with severely tethered mitral leaflet.32) A simple annuloplasty sometimes worsen the tethering of posterior leaflet and it induces recurrent MR33-36) and has a possibility to induce functional mitral stenosis.37-39) With these facts, additional procedures should be considered for patients with severely tethered valve or dilated left ventricle.
Submitral Procedure
Several submitral targeted surgical procedures for functional MR have been produced (Fig. 4).40-45) Relocation of PMs is effective for improving the mitral tethering; however, relocation of PM head for anterior leaflet alone has a possibility to remain the tethering of posterior leaflet. To overcome this problem, novel surgical procedure for functional MR named "papillary heads optimization" has been developed (Fig. 5). In this method, the PM head for posterior leaflet was connected to the PM head for anterior leaflet by using a polytetrafluoroethylene suture in each PM. After annuloplasty by a semi-rigid ring, its arms were re-suspended towards the mid-anterior mitral annulus and the sutures were passed through it and tied after the length adjustment. This method aims physiological relocation of both PM head for anterior leaflet and for posterior leaflet towards physiological position to avoid residual tethering of both leaflet after annuloplasty and prevention of further left ventricular remodeling by the sutures.
Visualizing the Subvalvular Structure
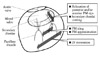
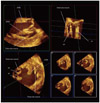
In the setting of submitral-targeted surgery such as papillary heads optimization, a detailed understanding of the submitral apparatus is essential for successful repair and for evaluating results of surgery. Adequate imaging is necessary for precise quantitative evaluation of the mitral valve structure. Three dimensional TEE has become widely available recently and it plays an essential role in understanding the status of the mitral valve apparatus. However, the conventional trans-left atrial view sometimes fails to visualize the subvalvular apparatus. On such occasion, transgastric approach should be employed.46) Transgastric view provides optimal image for quantitative analysis of the submitral apparatus (Fig. 6). A typical case which underwent papillary head optimization procedure is shown in Fig. 7. PM heads in each PM are clearly visualized. With the aid of quantitative software Real View (YD, Nara, Japan), these images allows accurate measurement of the pre and postoperative distance between PM head and mid-anterior annulus and the tenting volume, etc (Fig. 8). In this particular patient, the distance between posterior PM head for anterior leaflet and mid-anterior annulus were shorten from 26.5 mm to 23.5 mm during the surgery. On the other hand the distance between anterior PM head for anterior leaflet and mid-anterior annulus only changed from 24.1 mm to 24.0 mm. The distances between the mid anterior annulus and the both PM head for posterior leaflet were markedly reduced. Although MR was completely controlled in this patient, the colorized postoperative mitral leaflet by a Real View includes the red part in the lateral site, indicating slight residual tethering of leaflet in lateral site. Such information will assist further improvement of the quality of the surgery.
Summary
Three dimensional echocardiography plays an essential role to understand the geometry of mitral valve complex, including PM, PM head division, chordae tendineae, leaflets and annulus and contributes greatly to decision making of the surgical strategy in functional MR and its postoperative assessment.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download