Abstract
Isolated left ventricular noncompaction (LVNC) is a rare disorder caused by embryonic arrest of compaction. LVNC is sometimes associated with other congenital cardiac disorders; however, there have been few reports of its coexistence with a left ventricular aneurysm. A 40-year-old woman was admitted to our hospital for renal infarction. She had a history of embolic cerebral infarction 10 years ago. Transthoracic echocardiography showed prominent trabeculae and deep intertrabecular recesses which are filled with blood from the left ventricular (LV) cavity. A thrombus in the akinetic apical wall was confirmed by contrast echocardiography. Using cardiac computed tomography and magnetic resonance imaging, we rejected a possible diagnosis of suspicion of coronary artery disease. She was diagnosed LVNC with a thrombus in apical aneurysm. Here, we report the first patient in Korea known to have LVNC accompanying LV congenital aneurysm presenting with recurrent embolism.
Isolated left ventricular noncompaction (LVNC) is a congenital cardiomyopathy caused by a defect in endomyocardial morphogenesis.1) It is characterized by prominent trabeculation and deep intertrabecular recesses, resulting in thickened myocardium consisting of 2 layers-compacted and noncompacted myocardium.2) LVNC is occasionally combined with other congenital cardiac diseases3-6) and genetic cardiomyopathies.7) Its clinical manifestations include heart failure, thromboembolism, ventricular tachyarrhythmia, and sudden death.8) The risk of embolic complications was mostly associated with impaired systolic function.9) There were only a few cases about coexisting left ventricular aneurysm.10-12) We report a rare case of LVNC associated with left ventricular (LV) aneurysm, presenting with recurrent embolism.
A 40-year-old woman was admitted to our hospital for abdominal and lower back pain lasting for 10 days. In 1999, she had an embolic stroke of the right middle cerebral artery territory; however, after 2007, she was lost to follow-up and discontinued all medications, including antiplatelet and anticoagulant therapy. She did not smoke or drink alcohol. She had none of the classic cardiovascular risk factors such as diabetes or hypertension nor any family history of cardiomyopathy or heart failure. Her chest radiography and laboratory data, including B-type natriuretic peptide and cardiac enzymes levels, were unremarkable. An electrocardiogram revealed nonspecific ST changes and intraventricular conduction delay. Abdominal computed tomography, performed because of her abdominal pain, revealed left renal infarction. To evaluate for an embolic source, transthoracic and transesophageal echocardiography were performed. Two-dimensional echocardiography showed prominent trabeculations at the interventricular septum and the lateral wall. Color Doppler imaging revealed deep intertrabecular recesses filled by blood flowing from the ventricular cavity (Fig. 1). The ratio of maximal thickness of the noncompacted to compacted layers in the lateral wall was greater than 2 at end-systole. The 4-chamber and 2-chamber views defined the LVNC in the mid-septal and inferior walls and apical wall thinning (Fig. 2). Because wall motion of the apex was akinetic at end-systole and end-diastole, we confirmed the diagnosis as an apical aneurysm. Additionally, thrombus in the apical aneurysm was shown by contrast echocardiography (Fig. 3). The LV ejection fraction by modified Simpson's rule was 48% (LV end-diastolic volume 89 mL and LV end-systolic volume 49 mL) and the ratio of early diastolic mitral inflow velocity to early diastolic mitral septal annular velocity (E/E') was 7.62. We performed coronary computed tomography angiography and cardiac magnetic resonance imaging (MRI) to differentiate the aneurysm from post-infarction aneurysms. On computed tomography, there was no coronary stenosis (Fig. 4). Cardiac MRI showed two-layered appearance of trabeculated and compacted myocardium, it revealed a thin compacted layer preserving contractility and an apical aneurysm with akinetic motion at end-diastole and end-systole (Fig. 5). No late gadolinium enhancement was observed. We therefore diagnosed her with left renal infarction caused by LVNC, coexisting with an LV aneurysm. She was prescribed warfarin and has followed up uneventfully to date.
LVNC is a rare congenital cardiomyopathy characterized by multiple prominent trabeculations with deep intertrabecular recesses.1) An arrest of compaction of the developing myocardium is strongly suggested as the probable mechanism of LVNC.1)9) Recently, the American Heart Association classified LVNC as a primary genetic cardiomyopathy.13) In contrast, the European Society of Cardiology considers LVNC to be an "unclassified cardiomyopathy".14) Multiple diagnostic criteria for LVNC have been proposed on the basis of echocardiography and cardiac MRI findings. The echocardiographic criteria suggested by Jenni et al.15) have become widely accepted. They are as follows: 1) thickened myocardium with a 2-layered structure consisting of a thin compacted epicardial layer [C] and a much thicker, noncompacted endocardial layer [N] or trabecular meshwork with deep endomyocardial spaces (N/C ratio > 2.0 at end-systole); 2) predominant location of the pathology in the mid-lateral, mid-inferior, and apical areas; 3) color Doppler evidence of deep intertrabecular recesses filled with blood from the LV cavity; and 4) absence of coexisting cardiac abnormalities (in isolated LVNC). There have been many reports of coexistent congenital cardiac disorders, including atrial septal defect, ventricular septal defect, pulmonary stenosis, anomalous pulmonary venous connection, Ebstein's anomaly, and a bicuspid aortic valve.3-6) However, only a few cases of LVNC with LV aneurysm have been reported.10-12) The mechanism of aneurysm is uncertain. Sato et al.10) proposed impaired microcirculation of noncompacted and compacted layers as the mechanism of aneurysm formation in LVNC. However, in our patient, the epicardial coronary arteries appeared normal on coronary computed tomography angiography and neither perfusion defects nor delayed enhancement were seen on cardiac MRI. We therefore thought that our patient's aneurysm might be congenital rather than degenerative change of LVNC.
The classical triad of complications with LVNC is heart failure, ventricular arrhythmia, and systemic embolic events.8) Because there are limited data regarding treatment of this condition, it is recommended that clinical complications be managed according to the current guidelines for each clinical complication. Our patient presented with 2 embolic events: stroke and renal infarction. The prevalence of systemic embolic events in patients with LVNC varied in reports. Based on the high rate of embolic events reported in long-term follow-up data, Oechslin et al.8) recommended anticoagulant therapy for these patients, independent of ventricular systolic function. However, Oechslin and Jenni9) recently recommended anticoagulation therapy for patients with impaired systolic function (LV ejection fraction < 40%) because deep intertrabecular recesses and slow blood flow might increase the risk of thrombus formation. Our patient had a thrombus in an apical LV aneurysm. We believed that the apical thrombus was the embolic source of her presentation with renal infarction and that the apical aneurysm with slow blood flow was a risk factor for recurrent embolic events. Therefore, we suggest that anticoagulation therapy might be needed in patients with LVNC with coexisting LV aneurysm, even in the absence of systolic dysfunction or atrial fibrillation.
In conclusion, we described a rare case of LVNC with LV aneurysm presenting as recurrent thromboembolic events. We believe that careful evaluation of LVNC patients for coexisting heart abnormalities such as aneurysms is essential for making the best clinical decisions for their management.
Figures and Tables
Fig. 1
Transthoracic echocardiography. (A) A modified 4-chamber view showing an apical aneurysm (An) and prominent trabeculations (arrows) on the basal to mid-lateral wall. (B) Color Doppler image showing blood flow in the recess between trabeculae. (C and D) Apical level of short axis view showing trabeculations and perfusion in intertrabecular recesses on color Doppler imaging.
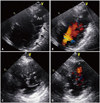
Fig. 2
Apical 4-chamber view (A and B) and apical 2-chamber view (C and D) in transthoracic echocardiography, showing increased trabeculations of the septal and inferior wall (arrows) and an apical aneurysm (An). The apical aneurysm of akinetic motion was defined at end-diastole (A and C) and end-systole (B and D). LV: left ventricle.
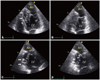
Fig. 3
Apical focusing image of apical long axis view (A) and apical short axis view (B) in contrast echocardiography, showing a thrombus (arrow) in the apical aneurysm. LV: left ventricle, An: aneurysm.
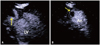
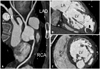
Fig. 4
Coronary computed tomography angiography, showing normal coronary artery (A), thrombus (arrow) in an apical aneurysm (B), and a thick noncompacted layer (C). LAD: left anterior descending artery, LCX: left circumflex artery, RCA: right coronary artery, LA: left atrium, LV: left ventricle, RV: right ventricle.
References
1. Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990. 82:507–513.

2. Ritter M, Oechslin E, Sütsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997. 72:26–31.

3. Burke A, Mont E, Kutys R, Virmani R. Left ventricular noncompaction: a pathological study of 14 cases. Hum Pathol. 2005. 36:403–411.

5. Attenhofer Jost CH, Connolly HM, Warnes CA, O'leary P, Tajik AJ, Pellikka PA, Seward JB. Noncompacted myocardium in Ebstein's anomaly: initial description in three patients. J Am Soc Echocardiogr. 2004. 17:677–680.

6. Betrián Blasco P, Gallardo Agromayor E. Ebstein's anomaly and left ventricular noncompaction association. Int J Cardiol. 2007. 119:264–265.

7. Biagini E, Ragni L, Ferlito M, Pasquale F, Lofiego C, Leone O, Rocchi G, Perugini E, Zagnoni S, Branzi A, Picchio FM, Rapezzi C. Different types of cardiomyopathy associated with isolated ventricular noncompaction. Am J Cardiol. 2006. 98:821–824.

8. Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000. 36:493–500.

9. Oechslin E, Jenni R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? Eur Heart J. 2011. 32:1446–1456.

10. Sato Y, Matsumoto N, Yoda S, Inoue F, Kunimoto S, Fukamizu S, Tani S, Takayama T, Tokai K, Kasamaki Y, Saito S, Uchiyama T, Koyama Y. Left ventricular aneurysm associated with isolated noncompaction of the ventricular myocardium. Heart Vessels. 2006. 21:192–194.

11. Ionescu CN, Turcot D. Left ventricular noncompaction and aneurysm revealed by left ventriculography. Catheter Cardiovasc Interv. 2011. [Epub ahead of print].

12. Yun H, Zeng MS, Jin H, Yang S. Isolated noncompaction of ventricular myocardium: a magnetic resonance imaging study of 11 patients. Korean J Radiol. 2011. 12:686–692.

13. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. American Heart Association. Council on Clinical Cardiology, Heart Failure and Transplantation Committee. Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups. Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006. 113:1807–1816.

14. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008. 29:270–276.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download