Abstract
Hemolytic anemia is recognized as a rare complication of mitral valve replacement or repair. We report on a 44-year-old man with shortness of breath and hemolytic anemia, 23 years after mitral valve replacement (Hall-Kaster), and a 63-year-old woman diagnosed of hemolytic anemia, 4 years after mitral and tricuspid annuloplasty (Tailor ring, An-core ring). Routine 2-dimensional transthoracic echocardiography revealed paravalvular leakage around the prosthesis. Subsequent real-time 3-dimensional (3D)transesophageal echocardiography helped the perceptional appreciation of the leakage and the measuring of the regurgitant orifice area using the anatomically correct plane. Surgical findings of each case fit those of 3D volumetric images.
Hemolytic anemia is one of the complications which may occur after mitral valve replacement or repair, mostly due to regurgitation around the prosthesis. We report two cases which qualitatively and quantitatively evaluated lesions of regurgitation around the mitral prosthesis using real-time 3-dimensional (3D) transesophageal echocardiography (TEE).
A 44-year-old male was referred to our cardiovascular center for anemia of unknown origin. About 23 years ago, he had had mitral valve replacement (Hall-Kaster prostheses, 27 mm) elsewhere, due to severe rheumatic mitral valve stenosis. Since then, he has received an oral anticoagulant. For the last 5 years, he has suffered with exertional dyspnea and dark-red-colored urine; for financial reasons, he refused further evaluation, and got only red blood cell (RBC) transfusions every 3-4 month and several medications from the cardiovascular out-patient clinic, including Carvedilol, Candesartan, and Digoxin.
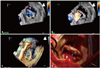
On physical examination, he had a pale conjunctiva and icteric sclera. There was a systolic murmur of grade VI/VI at cardiac apex; as well as a systolic ejection murmur at the right upper sternal border. Laboratory data showed white blood cell (WBC) 5020/mm3, hemoglobin 7.7 g/dL, hematocrit 27.4%, and reticulocyte 7%; serum iron 47 µg/dL, total iron-binding capacity 352 µg/dL, ferritin 14.21 ng/mL; LDH 4214 IU/L, total bilirubin 3.59 mg/dL, direct bilirubin 0.95 mg/dL. A peripheral blood smear revealed helmet cells and fragmented RBCs (schistocytes). His chest X-ray showed a markedly increased computed tomography ratio compared with the previous study three years ago. Echocardiography was performed with a high suspicion of mechanical hemolytic anemia related with mitral mechanical prosthesis. In the routine 2-dimensional (2D) transthoracic echocardiogram, significant paravalvular leakage was detected. There were significant rheumatic valvular pathologies of aortic valve steno-regurgitation and tricuspid valve regurgitation, with moderate resting pulmonary hypertension. Subsequent TEE revealed a well-preserved opening of the uni-leaflet tilting disc; however, there was significant paravalvular leakage along the outer-edge of the mechanical mitral prosthesis. A real-time 3D TEE showed an en-face view paravalvular defect (Fig. 1A), and 3D color Doppler demonstrated the dehiscence lesion combined with paravalvular leak during systole (Fig. 1B).
The surgical findings confirmed the paravalvular leak from the mid-posterior area of the mitral prosthesis, which then was covered with pericardium and 4 Ethibond sutures. Four months after a successful repair of mitral, aortic, and tricuspid valves, a postoperative 2D echocardiogram showed well functioning cardiac valves with no paravalvular leakage, normal left ventricular cavity dimensions, decreased wall thickness, and no resting pulmonary arterial hypertension. Even now, 14 months postoperatively, he is in good clinical condition, with a hemoglobin level of 14.9 g/dL, and does not require any transfusion.
A 63-year-old woman was referred to our cardiovascular division for further work-up of aggravated exertional dyspnea for one year. Four years ago, she had had mitral posterior annuloplasty (Tailor ring 27 mm), tricuspid annuloplasty (An-core ring), and a MAZE operation, due to severe mitral regurgitation, moderate tricuspid regurgitation, and moderate resting pulmonary hypertension, combined with atrial fibrillation. On physical examination, she was acutely ill-looking with a pale conjunctiva. There was a grade IV-V/IV systolic murmur at cardiac apex. Laboratory findings revealed WBC 5270/mm3, hemoglobin 6.8 g/dL, hematocrit 20.9%, and platelet 226000/mm3, LDH 3487 IU/L, total bilirubin 1.76 mg/dL, direct bilirubin 0.56 mg/dL, BUN/creatinine 26.3/1.66 mg/dL. A chest X-ray showed marked cardiomegaly with pulmonary edema. A conventional multiplane 2D TEE showed a para-ring tissue defect of posterior mitral annular ring & strangely located tricuspid annular ring which was widely apart from the native annulus. In real-time 3D TEE, both a para-ring tissue defect (5 O'clock) and tricuspid ring dehiscence were appreciated in the surgeon's view at one shot (Fig. 2). The mitral regurgitation was well demonstrated on live 3D zoom and 3D color Doppler (Fig. 3). The para-ring defect area was measured as 0.61 cm2 in the multi-planar rendering view (Fig. 5). Redo-mitral valve replacement (SJ mechanical 29 mm) and tricuspid annuloplasty (MC3 28 mm) were performed. Surgical findings fit the pre-operative 3D volumetric images (Fig. 4 and 5).
Hemolytic anemia is one of the potentially serious complications of prosthetic heart valves.1) Prosthesis-associated hemolysis can present clinically as anemia, heart failure, jaundice, dark urine, increasing serum lactate dehydrogenase, and a newly developed cardiac murmur.2) Peripheral blood smear shows variable numbers of schistocytes and small red cell fragments.
Mechanical hemolysis is associated with rapid acceleration and deceleration of the regurgitant jet with or without high peak shear rates.3) The incidence of significant hemolysis has decreased from 5-15% in the 1960-1970s to less than 1% in the 1990s, with the introduction of new-generation mechanical valves, which is resistant to structural deterioration.4-6) By nature, bioprosthetic valves and posterior rings are rarely associated with mechanical hemolysis, except in the presence of paravalvular leakage. As in the two cases presented here, the main mechanism currently for prosthetic heart valve associated hemolysis is paravalvular leakage.
Paravalvular leakage occurs mostly in the early postoperative period; intraoperative post-bypass TEE may encourage the surgeon to intervene immediately, before the patient leaves the operating room. Contrarily, the patients in these case reports presented and late paravalvular leaks diagnosed 23 and 4 years, respectively, after replacement or repair of the mitral valve, dramatically later than usual reports.7) Late-onset paravalvular leaks can be caused by suture dehiscence, localized infection, or focal distribution of mechanical shear forces. Clinical suspicion and exact diagnosis are important in these cases so as not to miss the chance of surgical correction.
To evaluate the prosthetic valves, TEE imaging has become the modality of choice, providing better image quality and resolution without the lung and bone tissue interference in conventional transthoracic echocardiography (TTE). By 2007, real-time 3D TEE using a fully-sampled matrix array transducer (X7-2t, 2.0-7.0 MHz, 2500 elements) offered 2D, Doppler, and 3D imaging in a single probe with excellent spatial resolution, ease of use, and time efficiency.8) 3D TEE provides excellent visualization of prosthetic mitral valves, permitting superior diagnostic accuracy as confirmed by surgical findings.9) As we presented here, routine TTE can diagnose regurgitation around valves but was insufficient to assess the degree or the anatomic shape. Using real-time 3D TEE, we observed real-time changes of the dehiscence around the prosthesis throughout the cardiac cycle, and at the same time could quantify the regurgitation area (Fig. 4). With the advance of technology, 3D TEE has become the modality of choice for evaluation of mitral prostheses, with fast acquisition and immediate online display during the peri-procedural10) or intra-operative periods.
One important pitfall when evaluating paravalvular leakage is the optimal gain setting. If the gain is too low, false-positive tissue defects around the mechanical prosthesis could appear. 3D color Doppler could differentiate true or false positives of dehiscence lesions as well as the nature of regurgitated jets of either paravalvular or valvular origin using various perspectives.
For the diagnosis of late paravalvular leakage, clinical suspicion and imaging modalities are crucial to lead surgical planning. We reported two cases of hemolytic anemia with paravalvular leakage evaluated by real-time 3D TEE, which might be useful in the perceptional appreciation and precise localization of the defect.
Figures and Tables
Fig. 1
A: A real-time 3-dimensional (3D) transesophageal echocardiography shows a paravalvular leak (arrows) outside of the mechanical mitral prosthesis (mid-posterior) in en-face view from a left atrial perspective, obtained in a single heart beat using 3D zoom mode. B: A full-volume 3D color volume reveals a significant systolic paravalvular regurgitant jet (arrows) and small physiological valvular regurgitant jets (arrowheads), acquired in 7 electrocardiography-gated cardiac cycles.
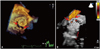
Fig. 2
This one 3-dimensional full-volume image shows the mitral posterior ring and peri-ring tissue defect (arrow) and the tricuspid annuloplasty ring prosthesis (asterisk) which was more than half detached from the anatomical annulus. ANT: anterior, POST: posterior, LAA: left atrial appendage, IAS: interatrial septum.
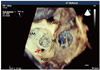
Fig. 3
A real-time 3-dimensional (3D) transesophageal echocardiography zoom image demonstrates a peri-ring tissue defect (arrow) of the mitral posterior ring (arrow) on surgeon's view in end-systole (A) and mid-diastole (B). A full-volume 3D color image reveals the regurgitant jet from the peri-ring defect (arrow), differentiated from valvular regurgitation (C); this view is similar to what the surgeon saw (D).
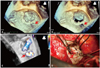
Fig. 4
Postprocessing of the live 3-dimensional transesophageal echocardiography volumetric image using the multi-planar rendering (MPR) mode in QLAB software (Philips Medical Systems, Andover, MA, USA) measures the peri-ring regurgitation orifice area using the anatomically correct plane as 0.61 cm2.
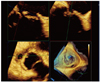
Fig. 5
A full-volume 3-dimensional (3D) color transesophageal echocardiography (TEE) image shows the tricuspid ring prosthesis in diastole (A), combined with regurgitation through the ring prosthesis during systole (B). A real-time 3D TEE zoom image reveals the tricuspid ring prosthesis and several thread-like strands caused by detachment from the native annulus (C), which revelation is confirmed in the surgical findings (D).
Acknowledgements
We express great thanks to the Korean Heart Foundation for their financial support of the first case's patient.
References
1. Edmunds LH Jr, Clark RE, Cohn LH, Grunkemeier GL, Miller DC, Weisel RD. Guidelines for reporting morbidity and mortality after cardiac valvular operations. Ad Hoc Liaison Committee for Standardizing Definitions of Prosthetic Heart Valve Morbidity of The American Association for Thoracic Surgery and The Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 1996. 112:708–711.

2. Maraj R, Jacobs LE, Ioli A, Kotler MN. Evaluation of hemolysis in patients with prosthetic heart valves. Clin Cardiol. 1998. 21:387–392.

3. Garcia MJ, Vandervoort P, Stewart WJ, Lytle BW, Cosgrove DM 3rd, Thomas JD, Griffin BP. Mechanisms of hemolysis with mitral prosthetic regurgitation. Study using transesophageal echocardiography and fluid dynamic simulation. J Am Coll Cardiol. 1996. 27:399–406.

4. Iguro Y, Moriyama Y, Yamaoka A, Yamashita M, Shimokawa S, Toyohira H, Taira A. Clinical experience of 473 patients with the omnicarbon prosthetic heart valve. J Heart Valve Dis. 1999. 8:674–679.
5. Skoularigis J, Essop MR, Skudicky D, Middlemost SJ, Sareli P. Frequency and severity of intravascular hemolysis after left-sided cardiac valve replacement with Medtronic Hall and St. Jude Medical prostheses, and influence of prosthetic type, position, size and number. Am J Cardiol. 1993. 71:587–591.

6. Mecozzi G, Milano AD, De Carlo M, Sorrentino F, Pratali S, Nardi C, Bortolotti U. Intravascular hemolysis in patients with new-generation prosthetic heart valves: a prospective study. J Thorac Cardiovasc Surg. 2002. 123:550–556.

7. Demirsoy E, Yilmaz O, Sirin G, Baran T, Tekin S, Sener D, Sonmez B. Hemolysis after mitral valve repair: a report of five cases and literature review. J Heart Valve Dis. 2008. 17:24–30.
8. Yang HS, Bansal RC, Mookadam F, Khandheria BK, Tajik AJ, Chandrasekaran K. American Society of Echocardiography. Practical guide for three-dimensional transthoracic echocardiography using a fully sampled matrix array transducer. J Am Soc Echocardiogr. 2008. 21:979–989. quiz 1081-2.

9. Sugeng L, Shernan SK, Weinert L, Shook D, Raman J, Jeevanandam V, DuPont F, Fox J, Mor-Avi V, Lang RM. Real-time three-dimensional transesophageal echocardiography in valve disease: comparison with surgical findings and evaluation of prosthetic valves. J Am Soc Echocardiogr. 2008. 21:1347–1354.

10. Yang HS, Srivathsan K, Wissner E, Chandrasekaran K. Images in cardiovascular medicine. Real-time 3-dimensional transesophageal echocardiography: novel utility in atrial fibrillation ablation with a prosthetic mitral valve. Circulation. 2008. 117:e304–e305.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download