Abstract
We describe a 42-year-old man presenting to the emergency department with cardiogenic shock. He had a prior history of acute pulmonary embolism (PE), and had been on anticoagulation for 2 years. Although computed tomographic pulmonary angiography performed at the emergency department showed no change in the extent of PE and did not support a role of surgical treatment, pulmonary embolectomy was recommended by attending physician based on clinical and echocardiographic hemodynamic findings like unstable vital sign and markedly enlarged right ventricle with severely depressed systolic function. Surgery confirmed the presence of fresh thrombi. After surgery, hemodynamic status was progressively improved, but the patient died due to pneumonia and pulmonary hemorrhage.
Pulmonary embolism (PE) is a relatively common cardiovascular emergency, and is associated with a high mortality.1) In this respect, early diagnosis of PE is clinically important. Because of the variable and nonspecific clinical presentations of PE, a diagnostic testing like computed tomographic pulmonary angiography (CTPA) is necessary to diagnose and determine the extent of disease. However, we sometimes experience a patient with a significant discrepancy between clinical findings and CTPA results. Here, we describe a patient with PE, in whom CTPA did not suggest the therapeutic role of surgical embolectomy. Notwithstanding, based on the clinical and echocardiographic judgment, the patient was referred to surgery, resulting in a progressive, hemodynamic improvement postoperatively.
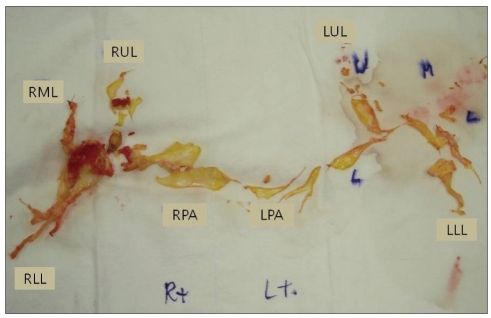
A 42-year-old man presented to the emergency department with recently aggravated severe dyspnea. The patient had been treated for Crohn's disease. He experienced acute PE 2 years ago and thereafter had been on warfarin, with a target INR of 2.0 to 3.0. The last follow-up CTPA that had been conducted 7 weeks before revealed a decreased extent of eccentric filling defects in descending braches of both pulmonary arteries, which was improved compared to the initial exam (Fig. 1A). Concomitant baseline echocardiography revealed mildly to moderately depressed right ventricular (RV) systolic function, mild tricuspid regurgitation (TR) and pulmonary hypertension with estimated systolic pulmonary arterial pressure of 62 mm Hg. The patient was alert and well oriented. His blood pressure was 81/70 mm Hg, pulse 89 bpm, respiration rate 40 breaths per minute, and body temperature 36.0℃ with oxygen saturation of 81% on room air. Serum level of cardiac enzymes was within normal ranges, but BNP was elevated at 396 pg/mL, and D-dimer was also elevated at 1,940 ng/mL. Serum level of hsCRP was within normal range. PT INR was 5.19. Initial arterial blood gas analysis showed combined metabolic acidosis and respiratory alkalosis (pH 7.237, pCO2 12.8 mm Hg, HCO3 9.6 mmol/L) and PaO2 of 126 mm Hg with supplemental oxygen at 10 liters/min via a partial rebreathing mask. Shortly after arrival at emergency department, he collapsed. Mechanical ventilation support for respiratory failure was initiated. Due to high clinical suspicion of acute PE, CTPA with venous phase imaging of lower extremities was conducted. But CTPA did not demonstrate any interval change in the extent of pre-existing chronic PE (Fig. 1B), and CT venography also did not demonstrate a source of pulmonary embolism. There was no lung parenchymal lesion which could explain the patient's acute respiratory failure. However, echocardiography revealed an increased RV size with severely depressed RV systolic function (Fig. 2A) in relation to D-shaped left ventricle (arrows in Fig. 2B), indicative of hemodynamically significant pulmonary hypertension and RV failure. Moderate TR was found with maximal velocity of TR jet of 3.58 m/sec, corresponding to an estimated systolic pulmonary arterial pressure of 71 mm Hg with an assumption of RA pressure of 20 mm Hg (Fig. 2C). Because of refractory respiratory failure unresponsive to maximum medical management, he was subsequently connected to a venovenous extracorporeal membrane oxygenation system in medical intensive care unit. Although CTPA did not support the role of surgical intervention, pulmonary embolectomy under cardiopulmonary bypass was performed on the third hospital day with a high probability of hemodynamically significant acute PE, based on clinical and echocardiographic findings. In the operating room, both pulmonary arteries were extensively inspected, and a large amount of organized thrombi with fresh thrombi were found in right and left pulmonary arteries extending to the segmental artery levels. The thrombi were removed successfully (Fig. 3) without unexpected events.
His hemodynamic parameters had progressively improved over the following 2 days, with a significant reduction in systolic pulmonary artery pressure to 40 mm Hg on invasive monitoring. The serial echocardiography also revealed improvements in RV systolic dysfunction (Fig. 2D) and the degree of interventricular septal flattening (Fig. 2E). However, pulmonary hemorrhage and ventilator associated pneumonia developed and he finally passed away on the 43rd postoperative day.
Since the first introduction of multi-detector CT, CTPA has become the method of choice for the diagnosis of suspected acute PE in everyday clinical practice.2) In hemodynamically unstable patients with acute PE, CTPA is strongly recommended because of its high sensitivity for detecting 'fresh' emboli in "large" pulmonary artery branches.3) However, there are two points that we should keep in mind in this setting. First, the predictive value of CTPA depends on the pretest probability of PE. Stein et al.3) have reported that in patients with a high pretest probability of PE, as assessed by the Wells score,4) a negative CTPA result had a low (60%) negative predictive value for PE, highlighting that clinical finding should be considered a high priority over CTPA findings in therapeutic decision-making. Second, in the setting of acute thromboembolic event on top of chronic PE, CTPA can underestimate the distribution of pulmonary emboli in the pulmonary branches. This is especially true when the emboli are linearly located along the pulmonary arterial wall or at the subsegmental artery level despite use of multi-detector CTPA.5) In addition, we suggest based on this case that in-situ thrombosis occurring slowly on chronic thromboemboli is difficult to detect with CTPA. As shown in Fig. 3, fresh thrombi were clearly demonstrated on operative findings, although CTPA could not suggest their presence. In this situation, clinical presentation of the patient must be discrepant from anatomic burden of pulmonary emboli. In this particular condition, echocardiography is able to guide therapeutic decision-making because it can reflect hemodynamic status of the patient, not simple anatomical thrombi burden.
Echocardiography is frequently performed in PE patients, because it permits reliable, qualitative, hemodynamic assessment of RV size, RV systolic function, and pulmonary arterial pressure.6)7) Prognostic information can be obtained with the help of echocardiography.8)9) Furthermore, in a critically ill patient with worsening symptoms who cannot undergo CTPA without delay, bedside echocardiography contributes to early diagnosis and treatment of PE. Kucher et al.10) have suggested that echocardiographic signs of RV pressure overload and dysfunction in a hemodynamically unstable patient with high pretest probability of PE may justify invasive treatment for PE.
In this case, worsened hemodynamic parameters on serial echocardiography helped us to apply an invasive approach for recurrent PE. Since the patient was on a venovenous extracorporeal membrane oxygenation system, intravenous thrombolysis was not considered an initial treatment option. Therefore, surgical embolectomy was performed, and an immediate hemodynamic improvement was achieved. This clinical decision was not supported by CTPA. As described above, CTPA can underestimate the burden of pulmonary thrombi, especially those that is distributed along the pulmonary arterial wall, at the subsegmental artery level, and in-situ thrombosis on top of chronic thrombi. Therefore, we believe that the present case is important and instructive in that hemodynamic assessment with echocardiography should be conducted to more accurately guide therapeutic strategy in clinically suspected PE patients.
References
1. Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006; 113:577–582. PMID: 16432055.
2. Schoepf UJ, Goldhaber SZ, Costello P. Spiral computed tomography for acute pulmonary embolism. Circulation. 2004; 109:2160–2167. PMID: 15136509.
3. Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, Leeper KV Jr, Popovich J Jr, Quinn DA, Sos TA, Sostman HD, Tapson VF, Wakefield TW, Weg JG, Woodard PK. PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006; 354:2317–2327. PMID: 16738268.
4. Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, Turpie AG, Bormanis J, Weitz J, Chamberlain M, Bowie D, Barnes D, Hirsh J. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000; 83:416–420. PMID: 10744147.
5. Henzler T, Barraza JM Jr, Nance JW Jr, Costello P, Krissak R, Fink C, Schoepf UJ. CT imaging of acute pulmonary embolism. J Cardiovasc Comput Tomogr. 2011; 5:3–11. PMID: 21051309.
6. Come PC. Echocardiographic evaluation of pulmonary embolism and its response to therapeutic interventions. Chest. 1992; 101:151S–162S. PMID: 1555480.
7. Lee JH, Park JH. Role of Echocardiography in Patients with Acute Pulmonary Thromboembolism. J Cardiovasc Ultrasound. 2008; 16:9–16.
8. Casazza F, Bongarzoni A, Capozi A, Agostoni O. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur J Echocardiogr. 2005; 6:11–14. PMID: 15664548.
9. Kurzyna M, Torbicki A, Pruszczyk P, Burakowska B, Fijałkowska A, Kober J, Oniszh K, Kuca P, Tomkowski W, Burakowski J, Wawrzyńska L. Disturbed right ventricular ejection pattern as a new Doppler echocardiographic sign of acute pulmonary embolism. Am J Cardiol. 2002; 90:507–511. PMID: 12208411.
10. Kucher N, Luder CM, Dörnhöfer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J. 2003; 24:366–376. PMID: 12581684.
Fig. 1
A: Baseline computed tomographic pulmonary angiography (CTPA) performed 7 weeks before the index visit to emergency department (ED). Transverse sections show chronic pulmonary embolism (PE) with eccentric filling defects (white arrows) in the right descending pulmonary artery and segmental artery of the right lower lobe. Multiple focal filling defects (arrowheads) are also seen in subsegmental arteries of the right lower and left lower lobes. B: CTPA performed immediately after the ED visit. No definite interval change is seen in the extent of pre-existing chronic PE.
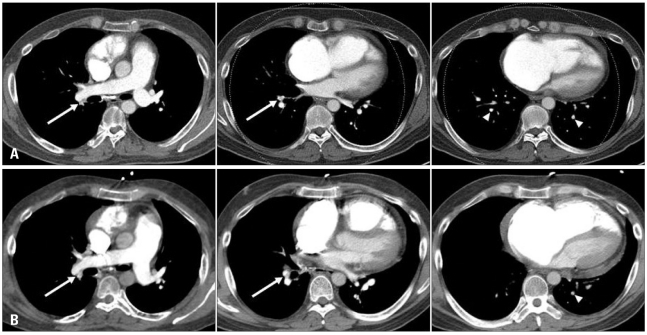
Fig. 2
A: Modified apical four-chamber view of the transthoracic echocardiography shows an enlarged right ventricular (RV) cavity with depressed RV systolic function. B: Parasternal short-axis view demonstrates an enlarged RV with flattening of the interventricular septum deviated to the left ventricle (white arrows). C: Tricuspid regurgitant jet with a maximum velocity of 3.58 m/sec. RA pressure was assumed to be 20 mmHg based on inferior vena cava response to respiration, resulting in an estimated pulmonary arterial systolic pressure of 71 mm Hg. D: Follow-up echocardiography performed on the 6th postoperative day shows improved RV systolic function and (E) improved degree of interventricular septal flattening (arrow).
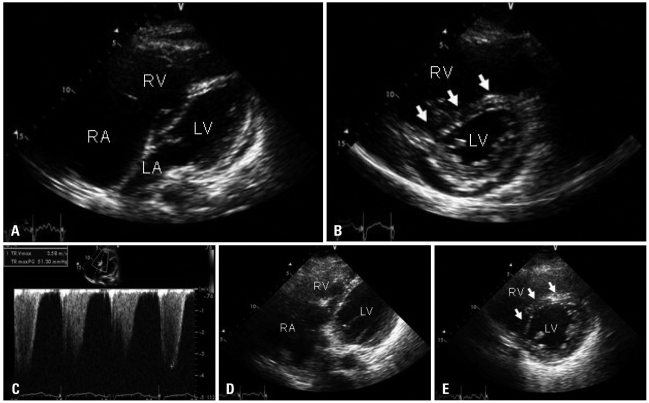
Fig. 3
Surgical specimen of pulmonary embolectomy. Whitish-yellow organized thromboemboli with fresh red thrombi are clearly shown. RPA: right pulmonary artery, RUL: right upper lobe, RML: right middle lobe, RLL: right lower lobe, LPA: left pulmonary artery, LUL: left upper lobe, LLL: left lower lobe.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download