Abstract
A 55-year-old man with massive pulmonary thromboembolism underwent thrombolysis, pulmonary artery embolectomy and tricuspid annuloplasty. Nine months later, a mobile echogenic intra-cardiac mass was found in the tricuspid valve. Because the patient had undergone annuloplasty, thrombosis was suspected as the most likely diagnosis and thrombolytic therapy was instituted. However, the size of the cardiac mass did not change and after surgical excision the mass was found to be a myxoma. Cardiac valvular tumors are uncommon and when they occur they are usually slow growing fibroelastomas. In this case, the rapid growing cardiac myxoma on the tricuspid valve was found after the occurrence of pulmonary thromboembolism. To our knowledge, this is first reported case of tricuspid valve myxoma in Korea.
Differential diagnosis of an intracardiac mass in the tricuspid valve after valvular surgery includes thrombosis, tumor, and vegetation, among others. Normal tissue such as fatty material and pectinate muscle surrounding the tricuspid annulus also may appear as a pseudo-tumor.1) In our case, because the patient had undergone annuloplasty, thrombosis was suspected as most likely and therefore thrombolytic therapy was instituted.2) Because the size of the cardiac mass did not change, however, we finally chose a surgical method and the mass was found to be a myxoma. To our knowledge, this is first reported case of tricuspid valve myxoma in Korea.
In November 2009, a 55-year-old man (who had smoking as a risk factor) was admitted to the hospital with progressive resting dyspnea. On physical examination, the patient was dyspneic and tachypneic. His jugular veins were distended. Initial laboratory work-up, including complete blood count, liver function tests, and cardiac enzyme level determination did not reveal any abnormality. He had an abnormal chest radiograph which showed cardiomegaly and a marked pulmonary trunk, and electrocardiogram reflected right ventricular strain pattern with normal sinus rhythm (Fig. 1). NT-pro BNP level was 5,592 pg/mL (normal: < 198 pg/mL), and D-dimer level was 2,296 ng/mL (normal: < 500 ng/mL). Transthoracic echocardiography (TTE) revealed right cardiac dysfunction with elevated right ventricular systolic pressure (RVSP). Pulmonary embolism was suspected and chest 3-dimensional computed tomography (3D CT) with contrast enhanced for pulmonary embolism showed a massive pulmonary thromboembolism (Fig. 2). No deep vein thrombosis in venous Doppler for the lower extremities or hypercoagulability in the laboratory test was apparent. Although the patient was hemodynamically stable, hypoxemia was noticed in arterial blood gas analysis (pH = 7.493, pCO2 = 27.9 mm Hg, pO2 = 61.9 mm Hg). Therefore he was thrombolysed with recombinant tissue plasminogen activator and experienced a rapid improvement in oxygenation.3) As follow-up, a second echocardiography and chest 3D CT was performed 7 days later. TTE showed impairment in right ventricular function even though RVSP was reduced from 119 to 96 mm Hg and a second CT showed a partially resolved status. There was multifocal extensive pulmonary thromboembolism in both pulmonary arteries (Fig. 3). Because further treatment was required to reduce pulmonary hypertension, we performed open pulmonary embolectomy with removal of thrombus from both pulmonary arteries, and De Vega's tricuspid annuloplasty to correct severe tricuspid valve regurgitation. The patient experienced no postoperative complications and made a satisfactory recovery. At 2 weeks after surgery, his TTE findings showed improvement in right heart function and RVSP (97 → 47 mm Hg). The patient was discharged from the hospital on warfarin. Nine months later, however, we found a mobile echogenic intra-cardiac mass (1.49 × 2.2 cm sized) at follow-up. The mass was attached to the septo-posterior leaflet portion of the tricuspid valve and protruded to the ventricular side. For differential diagnosis, trans-esophageal echocardiography was carried out and revealed a mobile mass attached to the annuloplasty groove (Fig. 4). Also his prothrombin time was occasionally inappropriate fluctuating from 1.58 to 2.65 for 9 months. Based on a suspicion of thrombus formation, we decided to do thrombolysis with tissue plasminogen activator (TPA). TPA (Alteplase®) was infused 100 mg, 10 mg as a bolus and 40 mg over 2 hours followed by 50 mg over 5 hours.2) However, the mass did not respond to thrombolysis, and the patient underwent surgery. Gross examination of the operative specimen revealed a polypoid mass with smooth surfaces; histological examination showed a myxoid matrix composed of an acid-mucopolysaccharide - rich stroma and embedded polygonal cells in hematoxylin and eosin stain, consistent with myxoma (Fig. 5).
Thrombosis is first suspected when an intracardiac mass is observed in the tricuspid valve after valvular surgery. Patients with a right atrial thrombus have several therapeutic options. For the patient at high risk for surgery, anticoagulation with heparin followed by an ongoing regimen of warfarin may be the treatment of choice.4) Thrombolytic therapy is also reported to be successful in some patients,5) although the possibility of pulmonary embolism caused by a fragmented thrombus due to thrombolytic therapy is a serious concern. In our case, given the patient's previous history of valvuloplasty, the valvular mass shown in TTE was suspected to be thrombus, so thrombolytic therapy was performed.2) After thrombolytic therapy, the size of the valvular mass did not change. We needed to exclude the possibility of other types of cardiac tumor and therefore decided to examine by surgical excision. The valvular mass was found to be myxoma. The differential diagnoses of intraatrial masses include atrial myxoma, thrombus related to atrial fibrillation, vegetation, metastatic tumors, and primary benign or malignant tumors.6) Although a mass usually shows up differently in an echocardiographic image, it is difficult to discriminate between thrombus and tumor. However, in our case it was difficult to make an accurate preoperative diagnosis. The patient underwent surgical excision of the tricuspid valvular mass. Histopathology allowed diagnosis of the mass as a tricuspid valvular myxoma.
Myxomas are the most common type of primary benign cardiac tumor, accounting for 50% of all primary cardiac tumors. Myxomas may develop in any chamber of the heart: approximately 75% of myxomas develop in the left atrium, 23% in the right atrium, and the remaining 2% in the ventricles. Primary tumors of the cardiac valves and chordae are uncommon. When they do occur, they are usually slow-growing fibroelastomas which manifest after several years.7) The interesting aspect of our case was the rapid growing cardiac myxoma which became apparent on the tricuspid valve within 9 months after tricuspid annuoplasty. To our knowledge, this is first reported case of tricuspid valve myxoma in Korea.
References
1. 2009 Training program for authenticated echocardiographist [Internet]. Korean Society of Echocardiography. 2009. cited 2011 Feb 21. Available from:
http://www.ksecho.org/infomation/contents/notice126/11_1.pdf.
2. Pierre-Justin G, Pierard LA. Management of mobile right heart thrombi: a prospective series. Int J Cardiol. 2005; 99:381–388. PMID: 15771917.
3. Piazza G, Goldhaber SZ. Fibrinolysis for acute pulmonary embolism. Vasc Med. 2010; 15:419–428. PMID: 20926501.
4. Porath A, Avnun L, Hirsch M, Ovsyshcher I. Right atrial thrombus and recurrent pulmonary emboli secondary to permanent cardiac pacing--a case report and short review of literature. Angiology. 1987; 38:627–630. PMID: 3631647.
5. Mügge A, Gulba DC, Jost S, Daniel WG. Dissolution of a right atrial thrombus attached to pacemaker electrodes: usefulness of recombinant tissuetype plasminogen activator. Am Heart J. 1990; 119:1437–1439. PMID: 2112880.
6. Reynen K. Cardiac myxomas. N Engl J Med. 1995; 333:1610–1617. PMID: 7477198.
7. Graça A, Nunes R, Costeira A, Almeida J, Bastos P. Cardiac papillary fibroelastoma of a mitral valve chordae revealed by stroke. Rev Port Cardiol. 1999; 18:937–939. PMID: 10590658.
Fig. 1
A: Chest X-ray showed cardiomegaly with right atrial enlargement and a prominent pulmonary trunk. B: Electrocardiogram showed characteristic features of RV strain with prominent S wave in lead I, Q wave in lead III, with T-wave inversion in lead III. RV: right ventricle.
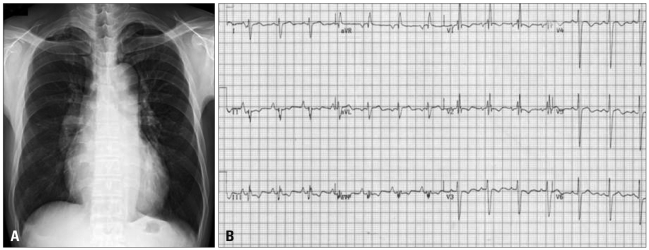
Fig. 2
A: Chest CT scanning detected a large thrombus in the right pulmonary artery. B: D-shaped LV was shown. C: Vmax was 4.96 m/s. Estimated right ventricular systolic pressure was 119 mm Hg (assumed right atrial pressure = 20 mm Hg, inferior vena cava dilatation and plethora was found). LV: left ventricle.
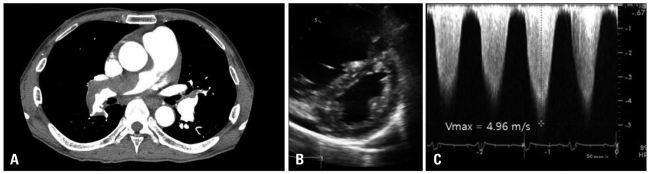
Fig. 3
A: A second computed tomogram showed a partially resolved multifocal extensive pulmonary thrombus. B: D-shaped LV remained after thrombolysis. C: Vmax was 4.46 m/s. After thrombolysis, estimated right ventricular systolic pressure was slightly reduced. LV: left ventricle.
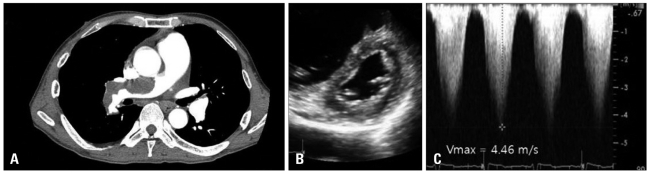
Fig. 4
A and B: Parasternal short axis view and apical four-chamber view. There was no visible mass in the immediate postoperative image. C and D: Parasternal short axis view and apical four-chamber view. There was a visible mass (arrow) with stalk 9 months later. E and F: Mid-esophageal RV inflow-outflow view in 64 degrees and mid-esophageal RV inflow view in 116 degrees. An intracardiac mass (arrow) was obvious (size: 2.2 × 1.5 cm2). After the 5th day of thrombolysis, the mass size remained unchanged. RA: right atrium, RV: right ventricle, RVOT: right ventricular outflow track, LA: left atrium, LV: left ventricle.
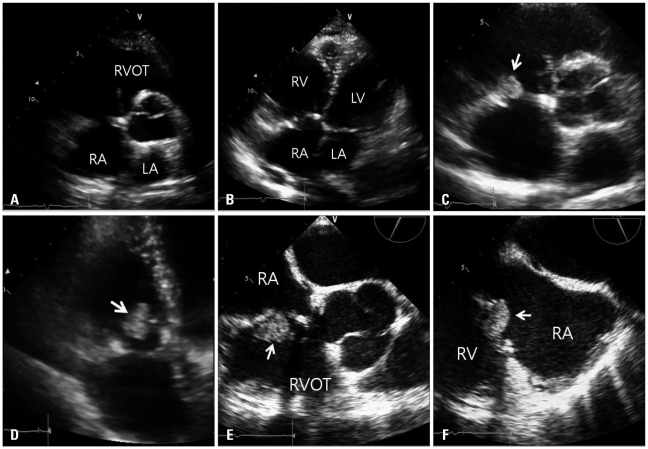
Fig. 5
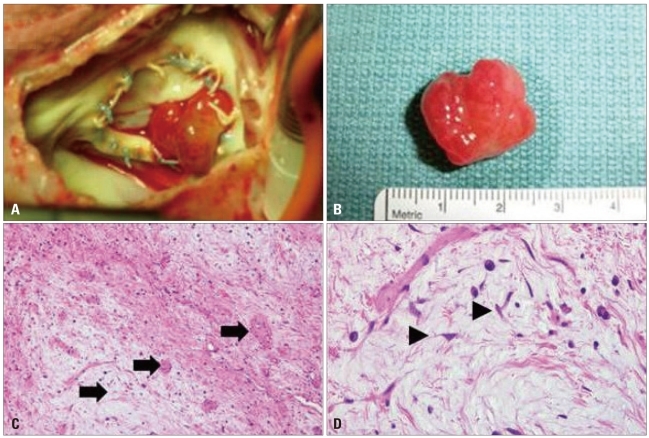
A: After right atrial opening, photograph showed a polypoid mass attached between annulus and tricuspid valve. B: After excision, mass size was similar to echocardiographically measured size (resected tumor size was approximately 2.5 × 1.5 cm2). C: Histological findings with Hematoxylin-Eosin staining. Histological examination of the mass revealed that it was composed of scattered small oval or stellate cells with abundant myxoid matrix, consistent with a diagnosis of nonmalignant cardiac myxoma. The tumor was hypocellular with prominent myxoid stroma and thick walled blood vessels (arrows, × 100). D: The tumor cells were elongated or stellate (arrowheads). They had round to elongated nuclei and eosinophilic cytoplasm. Lymphoplasma cells and histiocytes are present (× 400).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download