Abstract
Central venous stenosis or occlusion occurs in 11-50% of hemodialysis patients with prior subclavian vein cannulation and ipsilateral fistula or shunt. Most patients are asymptomatic but some require treatment to reduce the risk of thrombosis and improve inadequate hemodialysis pressure. In these cases, endovascular intervention, including ballooning and stenting, is a feasible strategy for selected patents. We report an unusual case of a 40-year-old man on hemodialysis that underwent endovascular stenting to treat right subclavian vein stenosis and experienced stent migration to the right ventricle, requiring surgical removal.
The main predisposing factor of central venous stenosis, a common complication of patients on hemodialysis, is previous insertion of a subclavian dialysis catheter, with an incidence rate as high as 50%.1-4) Treatment of subclavian vein stenosis aims to reduce the risk of thrombosis and improve upper extremity edema as well as improper hemodialysis pressure.5) Treatment for central venous stenosis includes percutaneous balloon angioplasty or stent implantation, and the latter is recommended because the former is followed by a rapid incidence of elastic recoil and restenosis.6) The elasticity and size variation of veins and arteries is an important consideration in choosing stent size and length. We report a case of stent migration to the right ventricle in a 40-year-old male patient.
A 40-year-old male patient that had been on hemodialysis for 12 years and suffered from chronic renal failure was transferred to our hospital with a chief complaint of dyspnea on exertion. One month earlier, his dialysis pressure of the outflow track had gradually increased during hemodialysis at the local hospital. The patient underwent venous angiography at same hospital and proximal right subclavian vein stenosis was diagnosed. To treat the lesion, percutaneous intervention was performed and a 14 × 60 mm Zilver self-expandable nitinol endovascular stent (COOK, Bloomington, IN, USA) was deployed in the right subclavian vein. Just after deployment of the stent, it migrated to the right ventricle. However, vital signs were stable and the patient was asymptomatic so another stent was successfully deployed by interventional radiologist. No further complications were reported and hemodialysis pressure of the outflow track improved. Therefore, no attempt was made to retrieve the migrated stent at the local hospital.
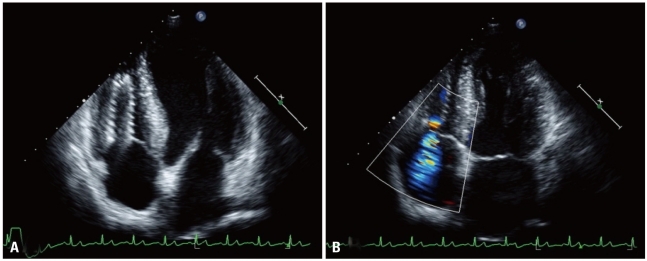
One month later the patient experienced new onset of dysppnea on exertion and was transferred to our hospital. On physical examination, his heart sound was regular, and a grade II pansystolic murmur was audible along both lower parasternal borders. There was no cardiomegaly upon chest X-ray but the right subclavian stent and another stent-like device was observed and located in the right side of the heart. A routine laboratory examination showed normal liver function test and mild anemia. Blood urea nitrogen and creatinine levels were 52 mg/dL and 10.0 mg/dL, respectively. Pro-brain natriuretic peptide concentration was 23,000 pg/dL. Transthoracic echocardiography showed 62% of left ventricular ejection fraction (EF) and trivial mitral regurgitation but the left ventricular enddiastolic dimension (LVEDD) was 59 mm and ratio of mitral E-velocity and E' velocity (E/E') was 12.4. Following examination of the right ventricle, a 4.55 × 1.72 cm coil-like structure with metallic echogenicity was observed. The migrated stent was seen at the right ventricular apex, and it spanned to the right atrium with significant tricuspid regurgitation (TR) flow on Doppler sonography (Fig. 1). Maximum velocity (Vmax) of TR was 260 cm/s. Echocardiography one year prior had shown no significant TR. In addition EF was 70%, LVEDD was 55 mm and E/E' was 8.7. Due to the risks of thrombosis, tricuspid valve injury, congestive heart failure, right ventricle perforation and cardiac arrhythmias, the patient was hospitalized and scheduled to undergo percutaneous intervention for stent removal.
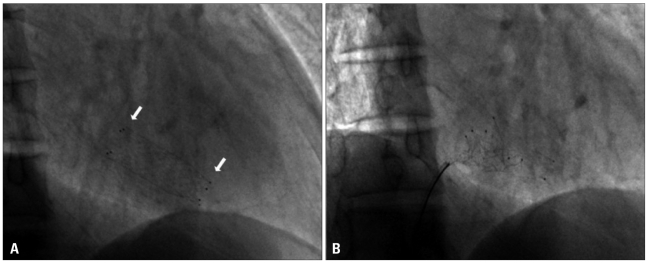
Fluoroscopy showed a metallic stent in the right ventricle (Fig. 2A) and another stent in the right subclavian vein through the superior vena cava. A vascular surgeon attended to remove the stent at the femoral vein level if it could not be removed through an 8 French sheath. We attempted to remove the stent from the right ventricle by a snaring technique with a 7 French XB guiding catheter (Cordis, Miami, FL, USA) introduced by a 8 French sheath after puncturing the right femoral vein. However, our attempt was unsuccessful (Fig. 2B), because even though were able to entrap the stent by snaring it, we were unable to pull it back through the guiding catheter. The stent was deformed and caught in the tricuspid valve so we had to terminate the procedure to avoid valve damage. Three days later, the endovascular stent was eliminated by open heart surgery (Fig. 3), and the damaged tricuspid valve was corrected by tricuspid valve posteroseptal commissuroplasty. Follow-up echocardiography showed no significant tricuspid valve stenosis and regurgitation. Vmax of TR was 236 cm/s, LVEDD decreased to 48 mm and EF reached 80% and E/E' was 10.8. Also, the symptoms of heart failure improved overall.
Complications of stent deployment include obstruction, recurrence due to intimal hyperplasia, vessel perforation, misplacement, and migration. Migration is rare but it can be life-threatening if the stent reaches the heart and pulmonary artery.7)8) Migration of stents from the superior vena cava to the innominate vein,9) right atrium,10) right ventricle,11-13) and pulmonary artery,14)15) after endovascular stenting for superior vena cava syndrome have been reported previously. Predisposing factors for stent migration in the condition of superior vena cava obstruction include 1) poor choice of lesion, 2) inadequate sizing of the stent, 3) inaccurate positioning of the stent, 4) effect of cardiac motion, 5) inaccurate vessel measurement, 6) cases in which the disease is expected to be resolved with treatment, for example, Hodgkin's lymphoma, 7) stent deployment system, and 8) delivery route.16)
If stent migration has occurred, the migrated stents have to be removed to prevent complications that include thrombosis, vessel trauma, and perforation. In case of migrated stent reached to the cardiac structures, it may cause myocardial injury resulting in arrhythmias, injury to valves, and papillary muscles, and rarely myocardial perforation causing hemopericardium and cardiac tamponade.17)18) The migrated stents can be managed either percutaneously or by open surgery. Taylor et al.16) described four different strategies for endovascular approaches of stent migration into the right atrium. These included: 1) snaring the stent directly, 2) angioplasty balloon-assisted snaring of the stent, 3) guide wire-assisted snaring of the stent, and 4) superior vena cava-to-inferior vena cava bridging stent. The primary objective of percutaneous management is to remove the stent, but if removal is not possible or failed, the stent should be fixed by additional stent at an alternative intravascular location to prevent repeated movements of the stent, which may cause vascular injury.17) Success rates of percutaneous techniques in the management of migrated stents exceed 90%.17)19) Open surgical methods to retrieve migrated stents are associated with high morbidity.15)20) Although the percutaneous management of migrated stents is highly effective, it is difficult in cases where the stents that have migrated to the right ventricle, and these cases may require surgical removal.13)19)
Our case was unusual because migration of the stent occurred during the intervention procedure to treat right subclavian vein stenosis, which leads to severe tricuspid valve regurgitation and congestive heart failure. Percutaneous stent removal was attempted, taking into consideration the patient's underlying disease and post-operative complications. We attempted to retrieve the stent by direct snaring, but had to switch to a surgical procedure because of technical difficulties and the possibility of additional damage to the tricuspid valve and other anatomical structures of the heart. If a migrated stent is entrapped in the heart and its valvular structure, percutaneous intervention may damage the heart structure and result in a fatal complication. In such cases, surgical removal is a safer and more feasible option, as shown in our case.
References
1. Schillinger F, Schillinger D, Montagnac R, Milcent T. Post catheterisation vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant. 1991; 6:722–724. PMID: 1754109.
2. Cimochowski GE, Worley E, Rutherford WE, Sartain J, Blondin J, Harter H. Superiority of the internal jugular over the subclavian access for temporary dialysis. Nephron. 1990; 54:154–161. PMID: 2314526.
3. Schwab SJ, Quarles LD, Middleton JP, Cohan RH, Saeed M, Dennis VW. Hemodialysis-associated subclavian vein stenosis. Kidney Int. 1988; 33:1156–1159. PMID: 2969991.
4. Surratt RS, Picus D, Hicks ME, Darcy MD, Kleinhoffer M, Jendrisak M. The importance of preoperative evaluation of the subclavian vein in dialysis access planning. AJR Am J Roentgenol. 1991; 156:623–625. PMID: 1781814.
5. National Kidney Foundation. NKF-DOQI clinical practice guidelines for hemodialysis adequacy. Am J Kidney Dis. 1997; 30:S15–S66.
6. Kovalik EC, Newman GE, Suhocki P, Knelson M, Schwab SJ. Correction of central venous stenoses: use of angioplasty and vascular Wallstents. Kidney Int. 1994; 45:1177–1181. PMID: 8007589.
7. Gray RJ, Dolmatch BL, Horton KM, Romolo JL, Zarate AR. Migration of Palmaz stents following deployment for venous stenoses related to hemodialysis access. J Vasc Interv Radiol. 1994; 5:117–120. PMID: 8136587.
8. Antonucci F, Salomonowitz E, Stuckmann G, Stiefel M, Largiadèr J, Zollikofer CL. Placement of venous stents: clinical experience with a self-expanding prosthesis. Radiology. 1992; 183:493–497. PMID: 1561356.
9. Verstandig AG, Bloom AI, Sasson T, Haviv YS, Rubinger D. Shortening and migration of Wallstents after stenting of central venous stenoses in hemodialysis patients. Cardiovasc Intervent Radiol. 2003; 26:58–64. PMID: 12522643.
10. Bartorelli AL, Fabbiocchi F, Montorsi P, Loaldi A, Tamborini G, Sganzerla P. Successful transcatheter management of Palmaz Stent embolization after superior vena cava stenting. Cathet Cardiovasc Diagn. 1995; 34:162–166. PMID: 7788697.
11. Poludasu SS, Vladutiu P, Lazar J. Migration of an endovascular stent from superior vena cava to the right ventricular outflow tract in a patient with superior vena cava syndrome. Angiology. 2008; 59:114–116. PMID: 18319233.
12. Srinathan S, McCafferty I, Wilson I. Radiological management of superior vena caval stent migration and infection. Cardiovasc Intervent Radiol. 2005; 28:127–130. PMID: 15602644.
13. Dubois P, Mandieau A, Dolatabadi D, Chaumont P, Gurnet P, Thiriaux J, Delcour C, Creplet J. [Right ventricular migration of a stent after endovascular treatment of a superior vena cava syndrome]. Arch Mal Coeur Vaiss. 2001; 94:1180–1183. PMID: 11794986.
14. Smayra T, Otal P, Chabbert V, Chemla P, Romero M, Joffre F, Rousseau H. Long-term results of endovascular stent placement in the superior caval venous system. Cardiovasc Intervent Radiol. 2001; 24:388–394. PMID: 11907745.
15. El Feghaly M, Soula P, Rousseau H, Chaiban F, Otal P, Joffre F, Cerene A. Endovascular retrieval of two migrated venous stents by means of balloon catheters. J Vasc Surg. 1998; 28:541–546. PMID: 9737466.
16. Taylor JD, Lehmann ED, Belli AM, Nicholson AA, Kessel D, Robertson IR, Pollock JG, Morgan RA. Strategies for the management of SVC stent migration into the right atrium. Cardiovasc Intervent Radiol. 2007; 30:1003–1009. PMID: 17605069.
17. Gabelmann A, Kramer S, Gorich J. Percutaneous retrieval of lost or misplaced intravascular objects. AJR Am J Roentgenol. 2001; 176:1509–1513. PMID: 11373221.
18. Prahlow JA, O'Bryant TJ, Barnard JJ. Cardiac perforation due to Wallstent embolization: a fatal complication of the transjugular intrahepatic portosystemic shunt procedure. Radiology. 1997; 205:170–172. PMID: 9314980.
19. Slonim SM, Dake MD, Razavi MK, Kee ST, Samuels SL, Rhee JS, Semba CP. Management of misplaced or migrated endovascular stents. J Vasc Interv Radiol. 1999; 10:851–859. PMID: 10435701.
20. Kadir S, Athanousilis CA. Athanasoulis CA, Green RE, Pfister RC, Roberson GH, editors. Percutaneous retrieval of intravascular foreign bodies. Interventional radiology. 1982. Philadelphia: WB Saunders;p. 379–390.
Fig. 1
A: Transthoracic echocardiography shows metallic coil-like structure in the right ventricle to right atrium through the tricuspid valve. B: Color Doppler image shows significant tricuspid regurgitation flow from the stent orifice.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download