Abstract
Pectus excavatum exists as varying anatomic deformities and compression of the right heart by the chest wall can lead to patient symptoms including dyspnea and chest pain with exertion. Echocardiography can be difficult but is critical to the evaluation and diagnosis of this patient population. Modifying standard views such as biplane transthoracic and 3-D transesophageal views may be necessary in some patients due to limitations from the abnormal anatomy of the deformed anterior chest wall. Apical four-chamber views when seen clearly can usually visualize any extrinsic compression to the right ventricle of the heart.
Pectus excavatum (PE), characterized by posterior displacement of the sternum and cartilaginous-rib attachments, is one of the most common congenital chest wall deformities.1)2) The physiological impact of PE varies. Symptomatic patients frequently complain of dyspnea with exertion, progressive loss of endurance, tachycardia, palpitations and chest discomfort.3-5) In a critical location, even small depressions of the chest can create significant cardiac dysfunction when the right heart and pulmonary outflow tract are compressed to varying degrees by the depressed sternum.5-7) An index of severity can be calculated by measuring the inner width of the chest (at the lowest level of the pectus defect) and dividing it by the distance between the posterior surface of the sternum (at the lowest part of the defect) and the anterior surface of the spine (with normal being approximately 2.52).2)3) In general, an index of 3.2 or greater is considered severe however symptoms do not necessarily correlate with severity of index.2-4) Echocardiography plays a significant role in the evaluation of patients with PE. The degree of right heart compression however is often difficult to assess by transthoracic echo (TTE) especially with severe deformities that prevent the operator from obtaining the normal transthoracic views.7-9)
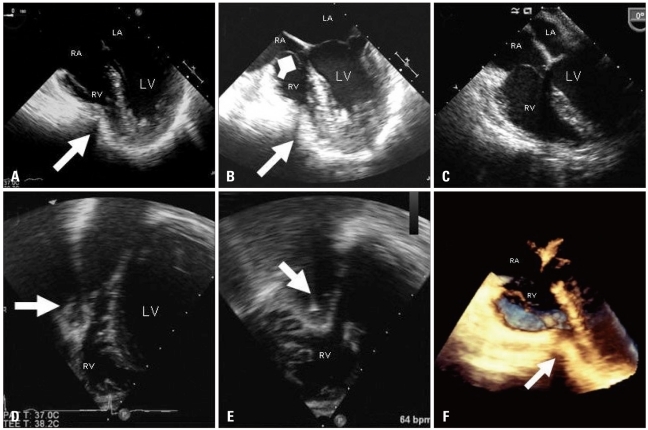
A 32-year-old woman presented to out-patient clinic for further evaluation of a 1-year history of progressive chest pain, fatigue, dizziness, paroxysmal tachycardia and dyspnea with exertion. With even mild exertional effort she experienced sharp, "stabbing" chest pain along the left lower and mid-sternum. A 12-lead electrocardiogram demonstrated a right-bundle branch block with left posterior fascicular block. She had a history of severe PE (index of > 4) for which she had undergone operative repair 18 months prior by an open resection of cartilage attachments and sternal "flip" as described by Hawkins & colleagues.10) Prior to her first correction, she had some dyspnea with exertion, however, cardiac work-up, including echocardiogram had been reported to not show any abnormalities. She noted onset of her current symptoms approximately 6 months after her PE repair. A subsequent operation with superficial anterior remodeling of the chest wall cartilage on the left side of the sternum had failed to relieve her progression of pain and symptoms. Current physical exam was normal with the exception of her chest wall. She exhibited post-operative abnormal remodeling of the chest wall with residual as well as some recurrent PE. The sternum protruded anterior with bilateral depression of the costal-sternal attachments creating a "wave-like" appearance (Fig. 1). A chest computed tomography (Fig. 2) and TTE (Fig. 3) were performed. Close attention to the anteroposterior planes (best seen from the apical four-chamber views) clearly demonstrated extrinsic compression and deformation of the lower mid-right ventricle (RV) by the chest wall which is more obvious during diastole (Fig. 3A) than systole (Fig. 3B). Biplane and live 3-D images of the preoperative transesophageal echocardiogram (TEE) improved the visualization and localization of extrinsic compression of the right ventricle (Fig. 5A, B, D-F). Tricuspid valve prolapse is seen likely due to partial compression of the RV resulting in distortion of the tricuspid annulus (Fig. 5B). There was no evidence for pulmonary hypertension. Open revision of her chest wall deformity was performed with placement of two stainless steel support bars (Lorentz Surgical, Jacksonville, FL, USA) and a trabecular metal implant (Zimmer, Inc., Minneapolis, MN, USA) which elevated the sternum and depressed regions 3-4 centimeters anterior to the RV with good cosmetic results (Fig. 4A and B). Post-operative TEE images showed resolution of extrinsic RV compression (Fig. 5C).
Symptomatic PE patients often present for cardiovascular evaluation with TTE being the most commonly utilized diagnostic modality. Documenting the effects of a depressed chest wall on the right heart and outflow tract as well as any associated interference with diastolic filling is critical for decision making in patient treatment and the need for potential surgical intervention.
Modifying standard views such as biplane transthoracic and transesophageal views may be necessary in some patients due to limitations from the abnormal anatomy of the deformed anterior chest wall. In some cases, RV compression is often difficult to assess by TTE especially with severe deformities that prevent the operator from obtaining the normal transthoracic window views. Technical tips for assessment of the RV include 1) narrowing the 2-D sector width to optimize only RV structures; 2) use of harmonic imaging and adjustment of gain and compression for good contrast and endocrinal edge detection; 3) making all measurements at end-diastole or the frame demonstrating the largest chamber dimension; and 4) for 2-D measurements, obtain all acquisitions during quiet respiration or full-expiration. Apical four-chamber views when seen clearly can usually visualize any extrinsic compression to the RV of the heart. Use of all three possible traditional acoustic windows (parasternal short-axis, apical and subcostal) may be necessary. In an effort to minimize foreshortening of the RV, the transducer can be positioned down an intercostal space and laterally until the RV apex is clearly seen. In some patients a transesophageal and transgastric view may be necessary to better evaluate the right heart chambers and RV outflow obstruction. Many patients with PE have associated alterations in RV morphology and function. Assessment for localized sacculation of the RV wall, global dilation of the ventricle, prominent trabeculae, and/or hypertrophy of the moderator band is important. Identifying abnormalities, including mitral valve prolapse and aortic root measurements are especially critical in suspected or confirmed cases of Marfan syndrome. Resolution of mitral valve prolapse with release of the chest wall entrapment is seen in more than half of patients after PE correction.7)11)12) More importantly, many patients improve their symptoms such as exertional dyspnea and chest pain which may be related to extrinsic compression of the RV and reduced preload.4)5)7) Further studies are needed to better understand the pathophyiology of symptoms in relation to cardiac and chest wall function in patients with PE.
In conclusion, echocardiographic evaluation is critical in patients with symptomatic PE to assess the degree of RV compression. We presented a case of a PE in a patient with symptoms including severe chest pain with exertion that was diagnosed with the expertise of echocardiography and subsequently surgically corrected. Subtle abnormalities in cardiac structure and chest wall compression may result in debilitating symptoms in a small population of patients.2-4) When astutely performed, echocardiography can accurately provide clinically relevant information about cardiac size and any hemodynamic compromise in patients with PE.
References
1. Heinle J, Sabiston DC Jr. Sabiston DC, Lyerly HK, editors. Congenital deformities of the chest wall. Textbook of surgery: the biological basis of modern surgical practice. 1997. 15th ed. Philadelphia: WB Saunders Co;p. 1888–1896.
2. Fokin AA, Steuerwald NM, Ahrens WA, Allen KE. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg. 2009; 21:44–57. PMID: 19632563.
3. Jaroszewski D, Notrica D, McMahon L, Steidley DE, Deschamps C. Current management of pectus excavatum: a review and update of therapy and treatment recommendations. J Am Board Fam Med. 2010; 23:230–239. PMID: 20207934.
4. Jaroszewski DE, Fonkalsrud EW. Repair of pectus chest deformities in 320 adult patients: 21 year experience. Ann Thorac Surg. 2007; 84:429–433. PMID: 17643611.
5. Jaroszewski D, Steidley E, Galindo A, Arabia F. Treating heart failure and dyspnea in a 78-year-old man with surgical correction of pectus excavatum. Ann Thorac Surg. 2009; 88:1008–1010. PMID: 19699946.
6. Park SY, Park TH, Kim JH, Baek HK, Seo JM, Kim WJ, Nam YH, Cha KS, Kim MH, Kim YD. A case of right ventricular dysfunction caused by pectus excavatum. J Cardiovasc Ultrasound. 2010; 18:62–65. PMID: 20706572.
7. Coln E, Carrasco J, Coln D. Demonstrating relief of cardiac compression with the Nuss minimally invasive repair for pectus excavatum. J Pediatr Surg. 2006; 41:683–686. discussion 683-6. PMID: 16567176.
8. Krueger T, Chassot PG, Christodoulou M, Cheng C, Ris HB, Magnusson L. Cardiac function assessed by transesophageal echocardiography during pectus excavatum repair. Ann Thorac Surg. 2010; 89:240–243. PMID: 20103244.
9. Mocchegiani R, Badano L, Lestuzzi C, Nicolosi GL, Zanuttini D. Relation of right ventricular morphology and function in pectus excavatum to the severity of the chest wall deformity. Am J Cardiol. 1995; 76:941–946. PMID: 7484836.
10. Hawkins JA, Ehrenhaft JL, Doty DB. Repair of pectus excavatum by sternal eversion. Ann Thorac Surg. 1984; 38:368–373. PMID: 6486951.
11. Raggi P, Callister TQ, Lippolis NJ, Russo DJ. Is mitral valve prolapse due to cardiac entrapment in the chest Cavity? A CT view. Chest. 2000; 117:636–642. PMID: 10712985.
12. Fonkalsrud EW. Open repair of pectus excavatum with minimal cartilage resection. Ann Surg. 2004; 240:231–235. PMID: 15273545.
Fig. 1
A: Patient clinical photograph at physical examination demonstrating physical abnormality of the post-operative chest. B: Preoperative chest roentgenogram shows abnormal bony projection of sternum posterior
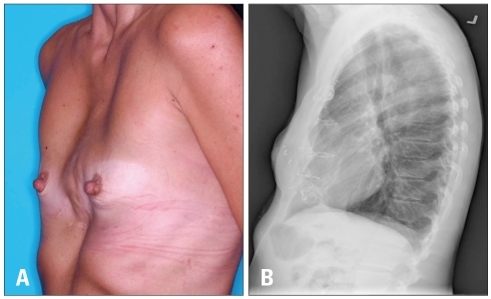
Fig. 2
Computed tomography with contrast showing bony projection of the deformed chest wall compressing the right ventricle (arrow).
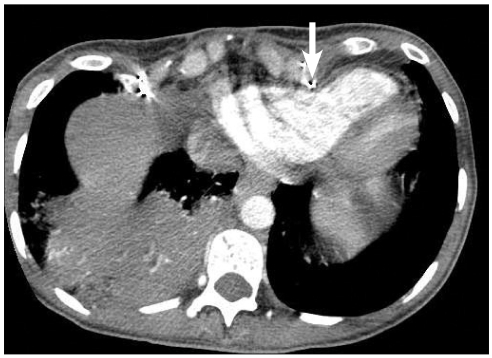
Fig. 3
Transthoracic echocardiography. Apical 4-chamber view demonstrates compression and deformation of the lower mid right ventricle by the chest wall in diastole which is more obvious during diastole (arrow) (A) than systole (B).
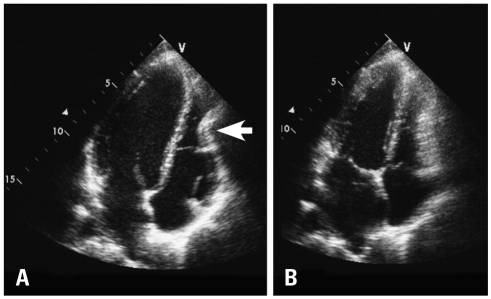
Fig. 4
A: Post surgical patient's chest was straightened and elevated 3-4 cm anterior to the heart and mediastinum with good cosmetic results and relief of cardiac compression. B: Lateral chest roentgenoram shows metal support bars and trabecular metal implant used to reconstruct patient's chest wall.
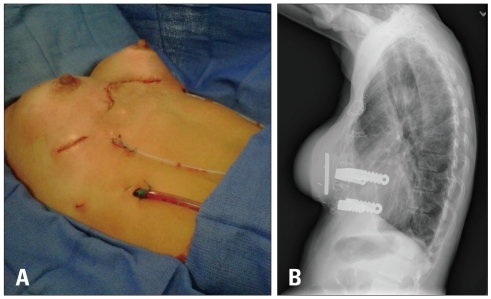
Fig. 5
Pre-operative transesophageal views showing extrinsic compassion of the RV during diastole (arrow) (A) and systole (arrow) (B). Tricuspid valve prolapse is evident during systole likely related to some distortion of the tricuspid annulus from extrinsic compression of the RV (square arrow) (B). Post-operative transesophageal image showing resolution of extrinsic RV compression (C). Note slight improvement in tricuspid valve prolapse as well following surgery. Pre-operative biplane images using three dimensional transesophageal probe from transgastric window showing extrinsic compression of the RV in the short axis (D) and long-axis views of the right ventricle simultaneously (arrows) (E). Note the acoustic shadowing behind the RV indentation due to bony structure. Improved visualization of the indentation of the right ventricle from live three dimensional image (arrow) (F). LV: left ventricle, LA: left atrium, RV: right ventricle, RA: right atrium.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download