Abstract
A 42-year-old male patient presented with refractory hypertension and congestive heart failure. He had taken hydrochlorthiazide 50 mg, carvedilol 25 mg, diltiazem 180 mg, and losartan 100 mg per day. Aortogram revealed a severe luminal narrowing in the distal thoracic aorta with a peak systolic pressure gradient of 60 mmHg across the lesion. Endovascular management was performed with 22 × 80 mm self-expandable Nitinol-S stent after predilation with 10 × 40 mm balloon. After endovascular management, the patient's blood pressure, left ventricular ejection fraction (LVEF) and dilated LV dimension were remarkably improved.
Coarctation of the aorta (COA) is a common congenital vascular disease which is the case that some of aorta is narrowed and is developed from just distal to the origin of left subclavian artery adhered by the aorta.1)2) However, middle aortic syndrome (MAS) is a rare condition affecting children and young adults. It is a clinico-pathological term that refers to isolated disease of the thoraco-abdominal aorta causing its segmental narrowing with concomitant stenosis of the renal and visceral branches. It is usually diagnosed in young adults, but may present in childhood as a challenging problem. Patients with MAS are often first detected due to refractory hypertension. Other later presentations include intermittent claudication, congestive heart failure, renal insufficiency and symptoms of hypertension associated end-organ damage. In some patients with MAS, the sign of congestive heart failure and refractory hypertension associated with left ventricular hypertrophy were remarkably improved after endovascular treatment.
A 42-year-old male visited our hospital with refractory hypertension. In the past, he has taken antihypertensive drugs for 2 months in spite of the hypertension diagnosed 16 years ago. He had taken hydrochlorthiazide 50 mg, carvedilol 25 mg, diltiazem 180 mg, and losartan 100 mg per day. He was alert and did not have an acute ill appearance. There were normal breathing sound in both lung fields and regular heart beats without murmur. We could not hear bruit on abdomen. The pulsation of the dorsalis pedis artery was weaker than that of the upper limb. His blood pressure (BP) was 208/122 mmHg at the upper extremities and 153/107 mmHg at the lower extremities. A simple chest X-ray showed cardiomegaly. An electrocardiography showed normal sinus rhythm with left ventricular hypertrophy. He was first diagnosed as dyslipidemia and type 2 diabetes in our hospital by laboratory exam. The results of erythrocyte sedimentation rate and C-reactive protein were 35 mm/hr and 3.3 mg/L. In the 2-D echocardiography, the left ventricular ejection fraction (LVEF) was 39% with global hypokinesia. LV mass index was 139.1 g/m2 and E/E' was elevated to 24.11. The LV end-diastolic dimension was 63 mm (Fig. 1A and D). There was accelerated abdominal aortic Doppler flow velocity with mosaic patterns in subcostal view, with a pressure gradient of 50 mmHg. A chest computed tomography (CT) angiography was checked to rule out the COA and revealed a stenosis of lower thoracic aorta at a diaphragmatic level (Fig. 2D). We also performed examination of other causes of secondary hypertension, but could not find other causes of high BP. The cardiac catheterization and stent implantation were planned. In the coronary angiogram, there was a significant stenosis in the proximal left coronary artery (LAD), the distal left circumflex artery (LCx) and chronic total occlusion in the distal right coronary artery (Fig. 3A and B). A stress test with 99mTc-tetrofosmin gated myocardial perfusion scintigraphy was performed to evaluate myocardial viability and showed a normal perfusion with global hypokinesia (Fig. 3C). In the aortogram, there was a critical luminal narrowing and the peak pressure gradient across the stenotic lesion was 60 mmHg (Fig. 2A and G). Then, the stenotic lesion was dilated with a 10 × 40 mm balloon catheter (Boston Scientifics, Washington, DC, USA) and a 22 × 80 mm self-expandable Nitinol-S stent (Taewoong Medical, Gimpo, Korea) was placed in the stenotic lesion. Additional ballooning was done using 14 × 40 mm balloon for more expansion of the stent. After ballooning, the peak pressure gradient across the stenotic lesion was decreased to 8 mmHg (Fig. 2H). Finally, the pulse of the dorsalis pedis artery was palpated normally, and there was no side effect such as an aortic dissection or an aortic aneurysm. The stent was placed successfully in the distal thoracic aorta on a follow-up angiogram and chest CT (Fig. 2B and E).
After the successful stenting, the BP of the upper limb turned stable at 120/80 mmHg. During hospitalization, the patient was able to reduce many anti-hypertensive agents due to stable BP at 120/80 mmHg. He was taking only one angiotensin converting enzyme inhibitor (imidapril 5 mg per day) and was getting better. He was discharged 1 week later without any problems. In a follow-up 2-D echocardiography after 2 months of stenting, the LVEF was 44% with improved wall motion except for hypokinesia of the apex of the anterior wall and E/E' was decreased to 11.43. The LV end-diastolic dimension was 60 mm (Fig. 1B and E). There was no accelerated abdominal aortic Doppler flow velocity with a pressure gradient of 5 mmHg. Six months later, the follow-up aortogram and CT angiogram findings showed the stent was placed well and his BP kept normal at 120/80 mmHg (Fig. 2C, F and I). In a 2-D echocardiography, the LVEF was 57% with more improved wall motion and more improved LV end-diastolic dimension with 55 mm (Fig. 1C and F). Furthermore, his LV mass index was decreased to 120.7 g/m2. At that time, we performed percutaneous coronary intervention for significant stenotic lesion of LAD and LCx because he had effort angina. He has been under clinical follow-up for 4 years as an out-patient without symptom.
In the cases of stenosis of thoracic or abdominal aorta, its cause can be rarely congenital, and it generates after curing aortitis.3) It also showed up, with Williams syndrome, congenital rubella syndrome, Takayasu's arteritis, and neurofibroma. Instead of "coarctation", it is also termed as "middle aortic syndrome" in aorta stenosis generating in lower thoracic or abdominal aorta.4) This patient has not had a particular infection, and inflammation indexes such as C-reactive protein, fibrinogen, erythrocyte sedimentation rate were within the normal ranges. This patient didn't meet the demands of diagnostic criteria of Takayasu's arteritis except for aortic stenosis. Therefore, we hardly considered Takayasu's arteritis as a causative disease. Considering refractory hypertension, dyslipidemia and diabetes, we can expect that a localized atherosclerosis gets worse as time goes by. As the gradient of pressure between aorta and femoral artery was growing, hypertension might have gotten worse. We may suspect that etiology of congestive heart failure was the thoracic aortic stenosis. And, interestingly, echocardiographic parameter of congestive heart failure such as LVEF and LV dimension were improved only two months after endovascular treatment of the stenosis of aorta dramatically.
The treatments of COA are traditional arterectomy with end to end anastomosis, graft surgery of subclavian artery, percutaneous transluminal angioplasty (PTA), and stent implantation. To minimize the side effects accompanied by PTA, treatment of COA has used the stent implantation, which can sustain luminal diameter regardless of the degree of intimal damage and reduce recoarctation. There is a recent report that stenting can be considered as a first treatment modality of COA instead of operation.1)2) It has reported that there were successful cases of stent implantation in stable patients with COA.5) We also reported the successful case of implantation in a patient with acute left heart failure and acute pulmonary edema with COA.
Balloon expandable covered stents are currently being developed that might reduce procedural complications. Instead of a balloon expandable stent, we deployed a self-expandable bare stent. It reduces procedural complications such as dissection and rupture of the aorta and needs only a small sized sheath (12F) with the additional ballooning. It has been reported that there were successful cases of stent implantation in stable patients with COA. Our case showed that the patient's sign of uncontrolled hypertension and congestive heart failure were remarkably improved after stenting, with no significant adverse cardiac events observed during 4-years of clinical follow-ups.
In conclusion, we have reported a rare case of middle aortic stenosis with coronary atherosclerosis and congestive heart failure associated with hypertensive cardiomyopathy. The signs and symptoms of congestive heart failure were rapidly, remarkably improved after endovascular treatment for aortic stenosis.
Figures and Tables
Fig. 1
2-D and M-mode echocardiography before stenting showed a decreased left ventricular ejection fraction and dilated left ventricular dimension (LV end-diastolic dimension was 63 mm) (A and D). In 2 month (B and E) and 6 month follow-up 2-D and M-mode echocardiography after stenting (C and F), the left ventricular ejection fraction and dimension were remarkably improved dimension (LV end-diastolic dimension was 60 mm, 55 mm respectively). LV: left ventricular.
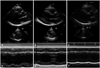
Fig. 2
Aortogram before stenting revealed significant luminal narrowing (arrow) at distal thoracic aorta (A), and after stenting (B) and 6 months follow-up after stenting (C) revealed remarkable improvement of luminal narrowing in the distal thoracic aorta. A chest computed tomography (CT) scan revealed coarctation (arrow) of the descending thoracic aorta (D). Chest CT scan after stenting (E) and 6 months follow-up chest CT scan after stenting (F) showed good apposition of the stent in the thoracic coarctation aorta. The peak pressure gradient across the stenotic lesion revealed 60 mmHg before stenting (G), it was decreased to 8 mmHg (H) after stenting and it was decreased to 0 mmHg 6 months after stenting (I).
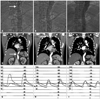
Fig. 3
In coronary angiogram, there was a significant stenosis in the proximal left coronary artery, the middle left circumflex artery and chronic total occlusion (arrow) in the distal right coronary artery. (A and B) A stress test with 99 mTc-tetrofosmin gated myocardial perfusion scintigraphy was performed to evaluate myocardial viability and showed a normal perfusion with global hypokinesia (C).

References
1. Harrison DA, McLaughlin PR, Lazzam C, Connelly M, Benson LN. Endovascular stents in the management of coarctation of the aorta in the adolescent and adult: one year follow up. Heart. 2001. 85:561–566.

2. Mahadevan V, Mullen MJ. Endovascular management of aortic coarctation. Int J Cardiol. 2004. 97:Suppl 1. 75–78.

3. Bergamini TM, Bernard JD, Mavroudis C, Backer CL, Muster AJ, Richardson JD. Coarctation of the abdominal aorta. Ann Vasc Surg. 1995. 9:352–356.

4. Fuster V, Alexander RW, O'Rourke RA. Hurst's the heart. 2001. 10th ed. New York: McGraw Hill;2390.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download