Abstract
Echocardiography is widely used to carry out non-invasive cardiac evaluation at the bedside and provides useful real-time information about hemodynamics. It can also be used to diagnose a stress-induced cardiomyopathy and its complications such as shock, heart failure and apical thrombus. Early diagnosis and management are important to prevent possible complications, and short-term follow-up by echocardiography can readily determine the improvement in these abnormalities. In this brief review, we summarize the role of echocardiography in stress-induced cardiomyopathy, with a special focus on its benefits in the era of new emerging diagnostic technology.
Stress-induced cardiomyopathy (also known as takotsubo cardiomyopathy or apical ballooning syndrome) is characterized by acute, reversible and transient left ventricular (LV) systolic dysfunction that resembles acute coronary syndrome but does not show significant stenosis on coronary angiography.1)2) Emotional and/or physical stressor can be the triggering factor. Excessive release of catecholamines due to stressors has been proposed to be involved in the pathogenesis of stress-induced cardiomyopathy because it causes structural changes of LV and disturbances in Ca2+ homeostasis.3) The recent body of evidence shows that postmenopausal women (90%) are predominantly affected by this syndrome and that stress-induced cardiomyopathy comprises 0.7-2.5% of cases of myocardial infarction (MI).4-6) Approximately 20% of complications were reported, including cardiogenic shock, heart failure, arrhythmias, intraventricular thrombus formation, LV free wall rupture, and even death.1)3)4)
Easy accessibility at the bedside of the hospitalized patient and the ability to use real-time noninvasive hemodynamic evaluation are the distinctive characteristics of echocardiography, particularly in the emergency setting. This review primarily addresses the role of echocardiography and emphasizes its usefulness in the diagnosis and management of this challenging disease.
The criteria for the diagnosis of stress-induced cardiomyopathy have evolved over the years. The most recently available criteria were proposed by the Mayo Clinic in 2008.7) The modified version of these criteria consists of four components: A) transient hypokinesis, akinesis, or dyskinesis in LV mid-segments with or without apical involvement; abnormalities in regional wall motion extending beyond a single epicardial vascular distribution; the presence (often, but not always) of a stress trigger; B) the absence of obstructive coronary disease or angiographic evidence of acute rupture of plaques that could be responsible for the observed wall motion abnormalities; C) new electrocardiographic abnormalities (ST-segment elevation and/or T-wave inversion) or modest elevation of levels of cardiac troponin in serum; and D) the absence of pheochromocytoma or myocarditis.
For the diagnosis of stress-induced cardiomyopathy, echocardiography is the most important imaging modality to distinguish this syndrome from acute MI, although coronary angiography is the best single tool to diagnose this unique cardiomyopathy. Abnormalities in LV wall motion show a regional or global pattern with a relative hypercontractile base in most cases. An inverted takotsubo pattern (mid-ventricular ballooning with sparing of the basal and apical segments) is a variant form.8)9) The dysfunction and regional wall motion abnormalities (RWMA) of the right ventricle (RV) (Fig. 1) are found in 30% of patients who tend to develop congestive heart failure and who have a poor outcome.10)11)
The modified Mayo criteria demand angiographic exclusion of coronary artery disease. In particular, coronary obstructive lesions must be immediately excluded in patients presenting with ST segment elevation. However, a recent report suggested the possible concurrence of coronary artery disease with stress-induced cardiomyopathy.12) Therefore, patients with coronary artery disease should not be excluded for the diagnosis of stress-induced cardiomyopathy if the coronary atherosclerosis is not significant or RWMA extend beyond single coronary artery distribution.
The unique morphology of stress-induced cardiomyopathy is apical ballooning and the relative compensatory hypercontractility of the basal segments. This phenomenon suggests that hemodynamics have an important role. That is, the degree of decreased LV ejection fraction (LVEF) and the existence of left ventricular outflow tract (LVOT) obstruction are the most important parameters in the evaluation and prediction of the severity and prognosis of stress-induced cardiomyopathy.
Initial LV function is usually impaired on hospital admission (mean LVEF, 20-49%) and, in general, resolve within days-to-weeks after initial presentation (mean period, 18 days).3) Most patients achieve a normal LVEF during hospitalization, but a few patients fail to reach the normal range.13) Moreover, a recent study revealed that absence of LV function recovery within 1 week (EF < 50%) was an independent factor associated with mortality.14)
An awareness of LVOT obstruction is an important factor in understanding hemodynamics in stress-induced cardiomyopathy. Basal hypercontractility is one of the characteristics, and can be aggravated with the use of inotropic agents such as dobutamine and dopamine. The Venturi effect around the LVOT results in the movement of the anterior mitral leaflets toward the interventricular septum in the systolic phase ["systolic anterior motion" (SAM)]. The reduction in forward flow contributes to the resultant low cardiac output. This effect may occur in up to one-quarter of patients presenting with a septal bulge associated with SAM and mitral regurgitation (MR).15) Other reports have confirmed structural abnormalities associated with LVOT obstruction, such as mid-ventricular septal thickening (particularly in elderly women).16) LVOT obstruction is a dynamic phenomenon depending on the hemodynamics at that time point, and thus echocardiography is a useful and readily accessible tool if unexplained hypotension or shock is observed. Apical five-chamber and parasternal long-axis views in two-dimensional (2D) images with color Doppler guidance can help in the evaluation of SAM severity. In the parasternal long-axis view, the M mode at the level of the mitral valve may give information about the relationship between the interventricular septum and anterior mitral leaflet.
MR can be observed with or without SAM.17)18) SAM can occur concomitantly with MR due to hemodynamic alteration, whereas the mechanism of MR without SAM may be different. The main factor involved in MR without SAM seems to relate to displacement of the papillary muscle, which leads to impaired leaflet coaptation secondary to tethering (Fig. 2). One study showed that patients with significant (moderate-to-severe or severe) acute MR had more depressed LVEF and a less complete and slower recovery of LV function.17) These findings imply that acute MR should be considered to be a potential marker of an adverse clinical course requiring aggressive treatment.
Atypical forms of stress-induced cardiomyopathy have increasingly been reported. Transient mid-ventricular ballooning with preserved basal and apical contractility (inverted takotsubo cardiomyopathy) (Fig. 3) has been described.9)19) The morphology of RWMA can be quite different, varying from a small area of akinesis limited to the LV apex to a large area of LV akinesis.20) Rare (but serious) complications such as LV free wall rupture and consequent death can occur in a manner similar to that seen in patients with MI.21)
New methods to measure systolic and diastolic dysfunction have recently been developed. Two-dimensional strain is useful to assess and quantify regional and global systolic function. It is based on tracking the movement of stable acoustic patterns ("speckles") within the myocardium frame-by-frame throughout the cardiac cycle.22) Patients with classical stress-induced cardiomyopathy show decreased longitudinal strain values from base to apex. In variant type of stress-induced cardiomyopathy, longitudinal strain is lowest at mid-LV segments. Despite the general perception of basal hypercontractility in stress-induced cardiomyopathy, total longitudinal strain of the LV base is also diminished in several segments at baseline.22)
Recent advances in transthoracic Doppler echocardiography allow non-invasive evaluation of coronary flow velocity and coronary flow reserve (CFR). There is a transient impairment of CFR in the acute phase of stress-induced cardiomyopathy, and this is closely correlated with LV systolic parameters.23)
Contrast echocardiography allows improved visual detection of the endocardial border (particularly the apical area). It is quite useful to use contrast echocardiography to exclude apical thrombi (Fig. 4).24) Contrast echocardiography can also demonstrate abnormalities in myocardial perfusion, which are indicative of microvascular dysfunction.25) Furthermore, normal myocardial perfusion pattern in the akinetic apex helps to discriminate stress-induced cardiomyopathy from anterior wall MI.
Low-dose dobutamine stress echocardiography (DSE) may be a useful and safe tool for the early prediction of myocardial viability in suspected stress-induced cardiomyopathy.26) However, high-dose DSE should be avoided because of the increased risk of induction of stress-induced cardiomyopathy.
Real-time three-dimensional (3D) imaging techniques allow nearly online quantification of the volume and mass of the left ventricle. In particular, rapid image acquisition is possible even in the absence of respiratory and electrocardiographic gating.27) If there are limitations in evaluation with transthoracic echocardiography due to poor windows, transesophageal echocardiography can provide clearer image quality. The relationship between MR and anatomic abnormalities of valves can be clearly observed. The hemodynamics of the LVOT can be easily understood with transesophageal echocardiography. Table 1 and 2 summarize the role of echocardiography in stress-induced cardiomyopathy.
There is no established consensus on how to manage stress-induced cardiomyopathy, but early detection of complications can protect against a poor outcome. In most cases, no specific therapy is required due to a favorable prognosis. If needed, diuretics are used to improve pulmonary edema. Although earlier reports suggested the usefulness of beta blocker in this patient population,7)28) a large scale registry data recently published could not find the protective effect of the simple beta blocking agent in preventing the occurrence or recurrence of stress-induced cardiomyopathy.13) Combined alpha- and beta-blocking agent may be advantageous, but this issue should be evaluated in the future.
RV involvement in stress-induced cardiomyopathy is relatively common and RV dysfunction is associated with lower LVEF, longer hospitalizations and more complications such as severe congestive heart failure, intra-aortic balloon pump, and cardiopulmonary resuscitation.10) In addition, Haghi et al.11) reported that pleural effusion was more common in patients with RV involvement and was predictive of RV dysfunction.
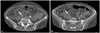
LV thrombus is a noteworthy complication and can occur both at initial presentation or at anytime later during the disease course.29) The intraventricular thrombus can be found not only in LV but also RV and left atrial appendage.13)30) The incidence of thrombus formation approximately results in about 2.5% of all the patients with documented stress-induced cardiomyopathy.31) An LV apical thrombus carries a great risk of cerebrovascular accident and distal embolization during the recovery phase of the LVEF (Fig. 5). Anticoagulants and heparin should be given on a short-term basis to patients with decreased LVEF, and short-term echocardiography follow-up is needed to evaluate other complications. Patients with SAM or LVOT obstruction should not be exposed to inotropic agents even if there are in shock.4)
Stress-induced cardiomyopathy is a syndrome with a wide spectrum of hemodynamics and variable prognoses. Echocardiography has many merits thanks to its non-invasiveness, portability, real-time accessibility, reproducibility and concurrent monitoring of anatomic and physiologic abnormalities using conventional (2D and Doppler imaging) as well as advanced diagnostic techniques (strain, tissue Doppler, contrast echo and 3D imaging). Repeat assessment is necessary to monitor recovery or possible complications, and to plan further treatment.
Figures and Tables
Fig. 1
Apical four chamber view shows ballooning on LV apex with RV involvement and biatrial enlargement (A). On color Doppler, moderate TR is detected (B) and peak TR velocity is 3.3 m/s with a derived systolic pulmonary arterial systolic pressure of 44 mmHg (C). LV: left ventricle, RV: right ventricle, TR: tricuspid regurgitation.
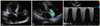
Fig. 2
Moderate mitral regurgitation detected by parasternal long axis view (A) and apical four chamber view (B).
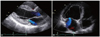
Fig. 3
Mid-ventricular ballooning with preserved basal and apical contractility is observed using echocardiography (A and B) and left ventriculography (C and D).
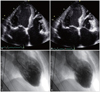
Fig. 4
Echocardiography shows a left ventricular (LV) apical thrombus on an apical four-chamber view (A) and contrast echocardiography confirms a filling defect of the LV thrombus (B). Follow-up echocardiography (C and D) shows no residual thrombus.
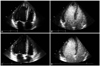
Fig. 5
Multiple thrombi (arrow) are detected in the right common iliac artery (A) and right external iliac artery (B) on abdominal CT.
References
1. Grawe H, Katoh M, Kuhl HP. Stress cardiomyopathy mimicking acute coronary syndrome: case presentation and review of the literature. Clin Res Cardiol. 2006. 95:179–185.

2. Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, Honda T, Hase M, Kai R, Morii I. Angina Pectoris-Myocardial Infarction Investigations in Japan. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina pectoris-myocardial infarction investigations in Japan. J Am Coll Cardiol. 2001. 38:11–18.

3. Nef HM, Mollmann H, Akashi YJ, Hamm CW. Mechanisms of stress (Takotsubo) cardiomyopathy. Nat Rev Cardiol. 2010. 7:187–193.

4. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006. 27:1523–1529.

5. Pernicova I, Garg S, Bourantas CV, Alamgir F, Hoye A. Takotsubo cardiomyopathy: a review of the literature. Angiology. 2010. 61:166–173.

6. Bybee KA, Prasad A, Barsness GW, Lerman A, Jaffe AS, Murphy JG, Wright RS, Rihal CS. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004. 94:343–346.

7. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008. 155:408–417.

8. Hurst RT, Askew JW, Reuss CS, Lee RW, Sweeney JP, Fortuin FD, Oh JK, Tajik AJ. Transient midventricular ballooning syndrome: a new variant. J Am Coll Cardiol. 2006. 48:579–583.
9. Van de Walle SO, Gevaert SA, Gheeraert PJ, De Pauw M, Gillebert TC. Transient stress-induced cardiomyopathy with an "inverted takotsubo" contractile pattern. Mayo Clin Proc. 2006. 81:1499–1502.

10. Elesber AA, Prasad A, Bybee KA, Valeti U, Motiei A, Lerman A, Chandrasekaran K, Rihal CS. Transient cardiac apical ballooning syndrome: prevalence and clinical implications of right ventricular involvement. J Am Coll Cardiol. 2006. 47:1082–1083.

11. Haghi D, Athanasiadis A, Papavassiliu T, Suselbeck T, Fluechter S, Mahrholdt H, Borggrefe M, Sechtem U. Right ventricular involvement in Takotsubo cardiomyopathy. Eur Heart J. 2006. 27:2433–2439.

12. Winchester DE, Ragosta M, Taylor AM. Concurrence of angiographic coronary artery disease in patients with apical ballooning syndrome (tako-tsubo cardiomyopathy). Catheter Cardiovasc Interv. 2008. 72:612–616.

13. Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, Haas TS, Hodges JS, Maron BJ. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol. 2010. 55:333–341.

14. Lee PH, Song JK, Sun BJ, Choi HO, Seo JS, Na JO, Kim DH, Song JM, Kang DH, Kim JJ, Park SW. Outcomes of patients with stress-induced cardiomyopathy diagnosed by echocardiography in a tertiary referral hospital. J Am Soc Echocardiogr. 2010. 23:766–771.

15. El Mahmoud R, Mansencal N, Pilliere R, Leyer F, Abbou N, Michaud P, Nallet O, Digne F, Lacombe P, Cattan S, Dubourg O. Prevalence and characteristics of left ventricular outflow tract obstruction in Tako-Tsubo syndrome. Am Heart J. 2008. 156:543–548.

16. Merli E, Sutcliffe S, Gori M, Sutherland GG. Tako-Tsubo cardiomyopathy: new insights into the possible underlying pathophysiology. Eur J Echocardiogr. 2006. 7:53–61.

17. Parodi G, Del Pace S, Salvadori C, Carrabba N, Olivotto I, Gensini GF. Left ventricular apical ballooning syndrome as a novel cause of acute mitral regurgitation. J Am Coll Cardiol. 2007. 50:647–649.

18. Haghi D, Rohm S, Suselbeck T, Borggrefe M, Papavassiliu T. Incidence and clinical significance of mitral regurgitation in Takotsubo cardiomyopathy. Clin Res Cardiol. 2010. 99:93–98.

19. Park HE, Kim JH, Yoon YE, Park JB, Lee W, Cho Y, Heo EY, Kim HK, Kim YJ, Sohn DW. A unique case of transient midventricular ballooning: an atypical manifestation of stress-induced cardiomyopathy involvoing both ventricles. Korean Circ J. 2008. 38:677–680.

20. Ibanez B, Benezet-Mazuecos J, Navarro F, Farre J. Takotsubo syndrome: a Bayesian approach to interpreting its pathogenesis. Mayo Clin Proc. 2006. 81:732–735.

21. Akashi YJ, Tejima T, Sakurada H, Matsuda H, Suzuki K, Kawasaki K, Tsuchiya K, Hashimoto N, Musha H, Sakakibara M, Nakazawa K, Miyake F. Left ventricular rupture associated with Takotsubo cardiomyopathy. Mayo Clin Proc. 2004. 79:821–824.

22. Heggemann F, Weiss C, Hamm K, Kaden J, Suselbeck T, Papavassiliu T, Borggrefe M, Haghi D. Global and regional myocardial function quantification by two-dimensional strain in Takotsubo cardiomyopathy. Eur J Echocardiogr. 2009. 10:760–764.

23. Meimoun P, Malaquin D, Benali T, Boulanger J, Zemir H, Tribouilloy C. Transient impairment of coronary flow reserve in tako-tsubo cardiomyopathy is related to left ventricular systolic parameters. Eur J Echocardiogr. 2009. 10:265–270.

24. Lee JW, Kim JY, Youn YJ, Sung JK, Lee NS, Lee KH, Yoo BS, Lee SH, Yoon J, Choe KH. Clinical characteristics and prognostic factors of stress-induced cardiomyopathy. Korean Circ J. 2010. 40:277–282.

25. Abdelmoneim SS, Mankad SV, Bernier M, Dhoble A, Hagen ME, Ness SA, Chandrasekaran K, Pellikka PA, Oh JK, Mulvagh SL. Microvascular function in Takotsubo cardiomyopathy with contrast echocardiography: prospective evaluation and review of literature. J Am Soc Echocardiogr. 2009. 22:1249–1255.

26. Uznańska B, Plewka M, Wierzbowska-Drabik K, Chrzanowski Ł, Kasprzak JD. Early prediction of ventricular recovery in Takotsubo syndrome using stress and contrast echocardiography. Med Sci Monit. 2009. 15:CS89–CS94.
27. Fujikawa M, Iwasaka J, Oishi C, Ueyama T, Park H, Yamamoto Y, Otani H, Iwasaka T. Three-dimensional echocardiographic assessment of left ventricular function in takotsubo cardiomyopathy. Heart Vessels. 2008. 23:214–216.

28. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004. 141:858–865.

29. Haghi D, Papavassiliu T, Heggemann F, Kaden JJ, Borggrefe M, Suselbeck T. Incidence and clinical significance of left ventricular thrombus in tako-tsubo cardiomyopathy assessed with echocardiography. QJM. 2008. 101:381–386.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download