Abstract
Carcinoid heart disease is characterized by heart valve dysfunction as well as carcinoid symptomatology. We report a case of carcinoid heart disease associated with a primary ovarian tumor. A 60-year-old woman presented for dyspnea evaluation with a history of facial flushing, telangiectatic skin changes, and pitting edema of both lower extremities. Chest radiography showed cardiomegaly, and echocardiography revealed an isolated, severe tricuspid regurgitation without left-sided valvular dysfunction. The tricuspid leaflets were severely retracted and shortened, resulting in poor coaptation. Furthermore, mild pulmonary valve stenosis and moderate regurgitation were found along with this deformation. The 24-hour urine analysis revealed an increased level of 5-hydroxyindoleacetic acid, and an ovarian tumor was apparent on computed tomography images. The mass was surgically removed, and the patient was diagnosed as having a primary ovarian carcinoid tumor. She was treated with chemotherapy and regularly followed-up with supportive treatments, deferring surgical correction.
Vasoactive substances from carcinoid tumors, which are uncommon malignancies arising from enterochromaffin cells, underlie the systemic manifestations of carcinoid syndrome. Primary ovarian carcinoid tumors are rare, accounting for 0.5% of all carcinoid tumors,1) and carcinoid syndrome and carcinoid heart disease are believed to complicate fewer than 10% of these rare ovarian neoplasms.2) Such tumors are usually suspected on detection of a pelvic mass and the presence of carcinoid syndrome, which is characterized by facial flushing and diarrhea, or typical carcinoid heart disease (right-sided valvular involvement).3) Most carcinoid tumors have to metastasize to the liver to result in symptoms of carcinoid syndrome; however, primary ovarian carcinoid tumors are unique in this regard because the venous drainage of the ovaries bypasses the portal venous system.4) We report the case of carcinoid heart disease associated with a surgical biopsy-confirmed primary ovarian carcinoid tumor.
A 60-year-old woman was admitted to our hospital with a three-month history of pitting edema of both lower extremities, worsening dyspnea on exertion, and easy fatigability. She had no specific cardiovascular risk factors except for hypertension. On physical examination, heart sounds were regular, and a grade III-IV pansystolic murmur was heard along the left lower parasternal border. In addition to pitting edema of the lower legs, facial flushing with telangiectatic skin changes was apparent in her face and legs. Routine laboratory examination including complete blood cell counts and liver or renal function tests did not reveal any abnormalities. Cardiomegaly was apparent on chest X-ray, and transthoracic echocardiography showed a normal left ventricular (LV) ejection fraction (56%), normal LV systolic and diastolic dimensions, and trivial mitral regurgitation. However, on right-sided heart evaluation, the tricuspid valve (TV) leaflet was severely retracted and shortened, and its chordae were also small and deformed (Fig. 1A and B). The TV leaflets showed very restricted movement and poor coaptation during the systolic phase. Accordingly, severe tricuspid regurgitation (TR) was noted on color Doppler imaging, and the TR velocity was measured at 2.7 m/s (Fig. 1C and D). Deformation was also apparent in the pulmonary valve (PV); the PV leaflets shrunk in size and had a motion limitation (Fig. 2A). Thus, moderate regurgitation was noted on color Doppler images (peak velocity, 1.5 m/s) (Fig. 2B and C). Together with regurgitation, color flow was accelerated through mild stenosed PV (peak velocity, 1.8 m/s) because of retracted PV annulus and stiffened leaflets (Fig. 2D, E and F). In addition to this valvular dysfunction, the right ventricle was enlarged, and apical contraction was depressed on echocardiography.
We sought systemic or hormonal etiologies for the exclusively right-sided valve involvement in this patient. The 24-hour urinary level of 5-hydroxyindoleacetic acid (5-HIAA) was measured, and abdominal computed tomography (CT) imaging was performed. On CT images, an intra-abdominal mass was detected on the right ovary (Fig. 3A), and we confirmed the increased level of 5-HIAA (68.2 mg/24 hr, normal range; 2.0-8.0 mg/24 hr) in 24-hour urine collection. The mass was surgically removed; it was 80 × 124 × 78 mm in size with an irregular multinodular shape and solid in nature (Fig. 3B). Histologically, the sections from the ovarian tumor displayed uniform, polygonal cells arranged in solid sheets with a trabecular pattern (Fig. 4A, 100×, hematoxylin-eosin staining). The trabeculae were lined with one to two layers of cells. The cells showed central, monotonous, and round nuclei with no mitoses or atypia (Fig. 4B, 400×). The amount of cytoplasm of the constituent cells was large, and it was strong acidophilic. Immunohistochemical analysis of the tumor cells showed strong granular cytoplasmic positivity for chromogranin A (Fig. 4C, 100×, chromogranin staining). Finally, the patient was diagnosed as having a primary ovarian carcinoid tumor. She was treated with adjuvant chemotherapy including paclitaxel and carboplatin and was subsequently discharged without any worsening complication. Treatment with diuretics and angiotensin converting enzyme inhibitors was continued to relieve right heart failure (HF) symptoms, with slight improvement of pitting edema and dyspnea. Follow-up echocardiography 3 months later showed no improvement of the valve deformity or TR severity; surgical correction, i.e., the replacement of the deformed valve using mechanical or bioprosthetics, was therefore considered to reduce right HF symptoms and to substantially delay the deterioration of right ventricular dysfunction. However, the patient wanted to defer surgical correction of the severe TR and to remain on conservative medical therapy because of the stated satisfaction with the clinical response and tolerability. Three years after diagnosis, she remains clinically stable; she has mild dyspnea on exertion in daily activities, and she is regularly followed-up at the outpatient clinic.
Carcinoid heart disease can manifest in more than half of patients with carcinoid syndrome. However, primary ovarian carcinoid tumors, accounting for 0.5% of all carcinoid tumors, are very rare, and fewer than 10% of primary ovarian carcinoid tumors are associated with carcinoid syndrome.1)2)
Patients with carcinoid heart disease exhibit fibrotic valvular plaques and myofibroblast proliferation that may cause valvular thickening and retraction leading to dysfunction.5) Most patients present with right-sided heart valve dysfunction because of the venous drainage of a vasoactive substance that is metabolized and secreted by the liver; left-sided valve dysfunction, which is usually accompanied by coronary vasospasm, is present as the result of a patent foramen ovale in less than 10% of individuals with carcinoid heart disease.6-8)
Despite an unusual manifestation of myocardial infiltrative pattern,9) echocardiography of patients with carcinoid heart disease has characteristic findings of thickened, shortened, and retracted TV and PV leaflets, changes that lead to incomplete coaptation, resulting in moderate or severe TV/PV regurgitation. Moreover, the PV is thickened and retracted, which also causes pulmonary regurgitation. Most findings can be explained by the systemic effects of 5-HIAA, which is activated via hepatic metabolism and drains into the right-sided heart system.8)10)11) Thus, importantly, severe TR alone without mitral valve involvement should raise suspicion of carcinoid heart syndrome as the cause of right-sided valvular heart disease.
There is no consensus on the appropriate treatment of carcinoid heart syndrome to improve mortality. In general, early detection and surgical removal of primary carcinoid tumors seems to be the only effective treatment that results in favorable clinical outcomes; two-year survival with surgical removal has been reported to be 40%, whereas that of medical treatment without surgery is only 8%.12) Because of systemic or hormonal effects, surgical removal would not be sufficient to treat carcinoid heart disease or to promote the regression of valvular lesions. With the recent decrease in valvular perioperative mortality, surgical correction of TV dysfunction could be generally undertaken, even in mildly symptomatic patients, to prevent further HF progression, right HF symptoms or signs, such as right ventricular enlargement or decline in right ventricular systolic function, or in anticipation of hepatic surgery.13) In the case of carcinoid syndrome with echocardiographic evidence of cardiac involvement, the three-year survival is reported to be 31%.14) Thus, individualized surgical correction of TV and right HF treatments are also needed to achieve better outcomes.
In conclusion, this case suggests that, in the differential diagnosis, we should consider a rare condition such as carcinoid heart disease; this condition is characterized by isolated, severe TR without significant left-sided valvular dysfunction, which could be easily assessed by transthoracic echocardiography.
Figures and Tables
Fig. 1
Echocardiographic imagings showing a deformed tricuspid valve with regurgitation. The echocardiogram on apical four-chamber view shows an enlarged right atrium and right ventricle. Both anterior and septal leaflets (arrows) reveal thickened and retracted (A). On the right ventricular inflow view, both anterior and posterior leaflets also show no mobility, shortening, and coaptation failure (B). Severe tricuspid regurgitation is noted on color Doppler imaging (C). Continuous-wave Doppler signal of tricuspid regurgitation shows an early peak velocity and a rapid decline, shaping dagger-shaped "V" wave (velocity, 2.7 m/s) (D). RV: right ventricle, RA: right atrium, LA: left atrium, TV: tricuspid valve, TR: tricuspid regurgitation.
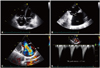
Fig. 2
Echocardiographic imagings showing a deformed pulmonary valve with regurgitation and stenosis. Parasternal short axis view of the pulmonary valve which has thickened and shortened leaflets (arrows); in addition, pulmonary annular contraction is present in zoomed imaging (A and D). On color Doppler imaging, moderate pulmonary regurgitation is shown and its peak velocity is at 1.5 m/s (B and C). Simultaneously, color flow is accelerated in systolic phase due to the stiffened and constricted leaflets (peak velocity, 1.8 m/s) (E and F). RV: right ventricle, Ao: aorta, PV: pulmonary valve, PR: pulmonary regurgitation, PS: pulmonary stenosis.
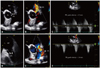
Fig. 3
Computed tomographic findings of carcinoid tumor. A large mass with inhomogenous density occupies the right pelvic cavity. The mass (white arrow) consists of multiple thick-enhancing septa and enhancing central soft tissue density (A). The right ovary is grossly enlarged; it is measured to be 80 × 124 × 78 mm in size and to weigh 461 g. The ovarian surface is smooth. The cut surface of the mass is bright yellow and solid, and it shows several foci of hemorrhage (B).

Fig. 4
Microscopic appearance of ovarian carcinoid tumor. Photomicrograph showing solid nests and broad trabeculae of tumor by cellular proliferation. These patterns were lined with one to two layers of cells (hematoxylin-eosin, 100×) (A). The regular round tumor cells have uniform rounded nuclei and abundant fibrovascular stroma (hematoxylin-eosin, 400×) (B). Immnuohistochemistry stains for neuroendocrine marker of the tumor cells reveals diffuse, strong, granular, and cytoplasmic staining (brown color) with chromogranin A (chromogranin, 100×) (C).

References
2. Stephan E, de Wit J. Carcinoid heart disease from primary carcinoid tumour of the ovary. Haemodynamic and cine coronary angiocardiographic study after operation. Br Heart J. 1974. 36:613–616.

3. Chaowalit N, Connolly HM, Schaff HV, Webb MJ, Pellikka PA. Carcinoid heart disease associated with primary ovarian carcinoid tumor. Am J Cardiol. 2004. 93:1314–1315.

4. Artaza A, Beiner JA, Gonzalez M, Aranda I, de Teresa EG, Pulpon LA. Carcinoid heart disease: report of a case secondary to a pure carcinoid tumour of the ovary. Eur Heart J. 1985. 6:800–805.

5. Simula DV, Edwards WD, Tazelaar HD, Connolly HM, Schaff HV. Surgical pathology of carcinoid heart disease: a study of 139 valves from 75 patients spanning 20 years. Mayo Clin Proc. 2002. 77:139–147.

6. Blick DR, Zoghbi WA, Lawrie GM, Verani MS. Carcinoid heart disease presenting as right-to-left shunt and congestive heart failure: successful surgical treatment. Am Heart J. 1988. 115:201–203.

7. Mansencal N, Mitry E, Forissier JF, Martin F, Redheuil A, Lepére C, Farcot JC, Joseph T, Lacombe P, Rougier P, Dubourg O. Assessment of patent foramen ovale in carcinoid heart disease. Am Heart J. 2006. 151:1129.e1–1129.e6.

8. Zuetenhorst JM, Bonfrer JM, Korse CM, Bakker R, van Tinteren H, Taal BG. Carcinoid heart disease: the role of urinary 5-hydroxyindoleacetic acid excretion and plasma levels of atrial natriuretic peptide, transforming growth factor-beta and fibroblast growth factor. Cancer. 2003. 97:1609–1615.
9. Kim YJ, Sohn DW, Bang YJ, Oh BH, Choi YS, Lee YW. Myocardial Involvement of Carcinoid Heart Disease: A Case Report. J Korean Soc Echocardiogr. 1998. 6:95–99.

10. Lundin L, Norheim I, Landelius J, Oberg K, Theodorsson-Norheim E. Carcinoid heart disease: relationship of circulating vasoactive substances to ultrasound-detectable cardiac abnormalities. Circulation. 1988. 77:264–269.

11. Møller JE, Connolly HM, Rubin J, Seward JB, Modesto K, Pellikka PA. Factors associated with progression of carcinoid heart disease. N Engl J Med. 2003. 348:1005–1015.

12. Møller JE, Pellikka PA, Bernheim AM, Schaff HV, Rubin J, Connolly HM. Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation. 2005. 112:3320–3327.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download