Abstract
Background
Conventional pacemaker implantation induces left ventricular (LV) dyssynchrony, which might affect the LV function. We sought to evaluate the impact of different right ventricular (RV) pacing sites on the LV dyssynchrony and performance.
Methods
Comprehensive echocardiographic evaluation including the atrio-ventricular, inter- and intra-ventricular dyssynchrony based on M-mode, conventional Doppler and tissue Doppler imaging (TDI) was done before and immediately after (< 7 days) pacemaker implantation. For the LV performance, LV ejection fraction, longitudinal peak systolic velocity at the mitral annulus (S') annular or mean longitudinal velocity of the 6 basal segments (Sm) were used. These results were compared with those of 15 age matched controls.
Results
A total of 79 patients (48 females, mean age 63 ± 12 years) underwent RV pacing at the apex (n = 45, group I) or the septum (n = 34, group II). After pacemaker implantation, the QRS duration was significantly increased in both groups, but the change was greater in group I (57.1 ± 28.3 versus 32.8 ± 40.5 msec). Both the S' and Sm were lower in pacing groups than those in controls and Sm was significantly higher in group II (4.2 ± 1.0 versus 4.9 ± 1.3 m/sec) than group I despite a similar LV ejection fraction. The aortic pre-ejection time and septal to posterior wall motion delay in patients with pacemaker were longer compared to normal controls, but there were no significant differences. Both the TDI velocity and strain analysis showed no difference of the dyssynchrony indices between the two groups, despite a higher tendency of Doppler strain dyssynchrony indices in the RV apical pacing group compared to those of the control.
Conclusion
Despite the marked increase of the QRS duration after pacing, M-mode, Doppler and TDI failed to demonstrate any difference according to the pacing sites. The long-term effect of the longitudinal contraction being less affected and a smaller increase of the QRS duration in the RV septal pacing group needs to be confirmed in a longitudinal follow-up study.
Right ventricular (RV) apical pacing allows for safe and stable long term pacing. However, RV apical pacing inevitably induces non-physiologic left ventricular (LV) activation with alteration of the intraventricular contraction sequence, which delays LV activation.1) This delay is accompanied with LV dyssynchrony, and the development of LV dyssynchrony was reported to be associated with deterioration of heart failure symptoms and the systolic LV dysfunction.2) To overcome this potential limitation, other pacing sites including the RV outflow tract, the interventricular septum, and the his bundle have been tried.3) However, contradictory results have been reported in the literature4)5) and the impact of different RV pacing sites on LV dyssynchrony has not been seriously investigated. In this study, we sought to evaluate the acute changes of LV dyssynchrony according to the RV pacing sites.
This study is a multicenter, prospective observational study. The study population consisted of the patients who needed permanent pacemaker implantation for sick sinus syndrome (SSS) or high degree atrio-ventricular (AV) block. Of these, we excluded the patients who had a temporary or permanent pacemaker prior to the study, and those with poor image quality, significant mitral or aortic steno-insufficiency, underlying left bundle branch block, or advanced LV systolic dysfunction (ejection fraction < 40%). Seventy nine patients with pacemaker implantation and 15 age-matched healthy controls were included.
Pacemaker leads were inserted through the subclavian vein using standard implantation techniques. The RV leads were positioned in the RV apex (n = 45) or interventricular septum (n = 34) under fluoroscopic guidance. The decision for the lead positioning was determined at the electrophysiologists' discretion.
All the patients underwent comprehensive echocardiographic evaluation before and after (< 7 days) pacemaker implantation. All the images were obtained with a standard ultrasound machine (Vivid 7, GE Vingmed, Horton, Norway) with a 2.5 MHz probe. Standard techniques were used to obtain M-mode, two-dimensional, and Doppler measurement in accordance with the American Society of Echocardiography guidelines. The tissue Doppler derived peak systole (S') were measured at the septal mitral annulus. The averaged peak systolic tissue velocity (Sm) was also calculated from 6 basal segments. S' and Sm were considered to represent the LV longitudinal function. The LV end-systolic, end-diastolic volume, and ejection fraction were calculated by the Simpson's methods from the apical four and two chamber views.
Digital loops with one cycle of fundamental 2D image and three cycles of the color coded tissue Doppler imaging (TDI) were acquired from a parasternal short axis view at the mid-papillary and three apical views for off-line analysis of LV dyssynchrony using Echopac (BT07, GE, Vingmed). All the images were transferred to one center and analyzed by one observer (GY Cho), who was blinded to the clinical data and the other echocardiographic information.
A delay in the LV ejection can be reflected in the LV filling time, which is measured by the mitral inflow velocity. The atrio-ventricular dyssynchrony was measure as the LV filling time as the ratio of the RR interval.6)
Using pulsed-wave Doppler, we measured the difference between the pre-ejection intervals from the QRS onset to the beginning of ventricular ejection at the right and left ventricular outflow tract.7)
A) M-mode echocardiography: The septal to posterior wall motion delay (SPWMD) was assessed using M-mode echocardiography at the parasternal window.8) The interval between the maximal thickening of the septum and posterior wall was calculated.
B) Conventional Doppler imaging technique: We measured the pre-ejection interval from the QRS onset to the beginning of ventricular ejection at the LV outflow tract by using pulsed-wave Doppler for the assessment of global intra-ventricular dyssynchrony.
C) Tissue Doppler imaging technique: The peak myocardial velocity during the ejection phase and the time to the peak myocardial velocity (Ts) were measured with reference to the QRS complex. If the peak velocity could not be defined because of the noise signal or flat velocity contour, then the sample volume (12 × 6 mm) was gradually moved to the apex or base until clear signal intensity could be obtained. Intra-ventricular dyssynchrony was assessed by measuring the difference of Ts between the basal septum and basal lateral segment (Ts-SL)9) or by measuring the standard deviation of Ts of 12 basal and mid segments (Ts-SD).10) For strain analysis, the longitudinal strain curves were obtained from 12 basal and mid segments. The region of interest with a 12 × 6 mm oval shaped was placed in the middle of the respective segments from the three apical views and maintained same position during the cardiac cycle by manually tracking to avoid blood or pericardial contamination. The minimal frame rate was 130 frames per second. The time to peak strain (Tε) with reference to the QRS complex were measured. The time difference of Tε between basal septum and basal lateral segment (Tε-SL) or standard deviation in time to peak strain among the 12 segments (Tε-SD) was obtained for the strain derived dyssynchrony.11) The timing of events, such as aortic valve opening and closure, was obtained from color-coded M-mode of anterior mitral valve from the apical windows.12)
D) 2D speckle strain: Radial strain using speckle tracking was assessed on LV short axis at the mid-papillary muscle level (frame rate varied from 60 to 80 frames per second). Endocardium was traced manually at the end-systolic frame. The traced endocardium was automatically divided into 6 segments. The strain curves for each segment were constructed. We measured the time to peak radial strain of each segment. The absolute time interval of peak strain between anteroseptum and posterior segment was calculated.13) In addition, the time interval between the earliest and latest segment (maximal temporal difference) was also measured.
Data are presented as the mean ± standard deviation for continuous variables and as proportion for the categorical variables. The mean values of continuous variable were compared by t-test or ANNOVA, and the differences in the prevalence between the groups were compared via χ2-test. All the analyses were performed with SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) and p < 0.05 was considered to be statistically significant.
The baseline characteristics and echocardiographic measurements are summarized in Table 1. Age, pre-pacing QRS duration and LV ejection fraction were comparable between the two groups (Table 1). After pacemaker implantation, LV volume and ejection fraction did not significantly change. The QRS duration was significantly increased in both groups after pacing, but the difference between the pre- and post-pacing QRS duration was significantly higher in apical pacing group (57.1 ± 28.3 versus 32.8 ± 40.5 msec).
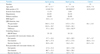
The echocardiographic variables immediately after pacemaker implantation are demonstrated in Table 2. The patients with RV apical pacing showed a lower S' (5.3 ± 1.3 versus 5.7 ± 1.5 cm/sec) and Sm (4.2 ± 1.0 versus 4.9 ± 1.3 cm/sec) than those with septal pacing. Aortic pre-ejection time and SPWMD in patients with a pacemaker were longer compared to those of normal controls, but there was no significant difference. Both TDI velocity and strain analysis showed no difference of dyssynchrony indices between the two groups, despite that there was a higher tendency of Doppler strain dyssynchrony indices in RV apical pacing group compared with those of the control (p = 0.063 for Tε-SL and p = 0.026 for Tε-SD).
In this study, we demonstrated that both septal and apical pacing produce LV dyssynchrony, but septal pacing is superior to RV apical pacing in terms of LV longitudinal function. However, there was no significant difference in LV dyssynchrony between septal and apical pacing.
In the past several years, there has been increasing recognition of the deleterious clinical effects of RV apical pacing, both in patients with pacemakers and in those with ICDs. In patients with a permanent pacemaker, every 1% incremental of RV pacing increases the risk of atrial fibrillation by 1% and the risk of heart failure hospitalization by 5.4%.14) Several studies have reported that RV apical pacing is associated with regional perfusion defects,15) adverse LV remodeling,16) a decrease in LV ejection fraction,17-19) and heart failure.2) More recently, several studies have reported that dyssynchronous LV contraction results from RV apical pacing.2)20)21)
The DAVID (Dual Chamber with VVI Implantable Defibrillator) trial suggested that RV apical pacing was associated with an increased risk of death and hospitalization for heart failure in patients with an implantable defibrillator.22) In that study, a higher cumulative percent of ventricular pacing was manifest in a significantly prolonged QRS duration at 6 months after pacemaker implantation and therefore, RV pacing might produce electrical dyssynchrony. Dual-chamber minimal ventricular pacing, as compared with conventional dual-chamber pacing, reduces ventricular desynchronization and moderately reduces the risk of persistent atrial fibrillation in patients with sinus node disease.23) The cumulative percent of ventricular pacing is associated with heart failure hospitalization and atrial fibrillation.14)22) Furthermore, in the patients with SSS, DDD pacing but not AAI pacing induces significant LV desynchronization and reduction of LV ejection fraction.24) Therefore, unnecessary RV pacing induces dyssynchronous LV contraction, which results in deterioration of LV systolic function and therefore, it can induce clinical heart failure. In our study, a dramatic increase of the QRS duration and SPWMD immediately after pacemaker implantation was demonstrated, and this suggests the potential detrimental long-term effect on the LV performance. Although the LV EF did not change immediately after implantation, the development of heart failure might depend on the pacing duration and so longer clinical observation is warranted.
By speckle tracking analysis, more than 50% of the patients showed dyssynchrony after RV pacing, which results in deteriorated LV systolic function and a worsened NYHA functional class.21) The development of LV dyssynchrony after permanent pacing is an important mechanism of LV function deterioration.25) Therefore, an alternative pacing mode or alternative pacing sites have been tested in order to prevent LV dyssynchrony and hemodynamic deterioration. Several studies have showed that either RV outflow tract (RVOT) pacing26-28) or RV septal pacing29)30) might have an advantage over classic RV apical pacing, but controversial results have also been reported.4) We could not demonstrate any significant difference of the LV dyssynchrony indices between the RV apical and septal pacing. According to the PROSPECT trial, no single echocardiographic measure of dyssynchrony may be recommended because of the poor reproducibility and moderate sensitivity of cardiac resynchronization therapy response.31) In this study, we used various kinds of mechanical dyssynchrony parameters. However, none of the echocardiographic measures of dyssynchrony showed a significant difference according to the pacing site.
One interesting finding of our study is that RV septal pacing showed better longitudinal systolic movement than did RV septal pacing. Although the resting LV EF was similar between the groups, this difference might affect the long-term LV performance, which should be tested by another study.
The LV mechanical function and dyssynchrony could be evaluated by a recently introduced speckle-tracking imaging technique, which might provide other indices including LV twist and 2-dimensional radial strain.32) Using this relatively new technique, it might be interesting to test whether LV mechanical function or dyssynchrony indices show significant difference according to the different pacing sites. Finally, although longitudinal function was better in the septal pacing group, we could not rule out the possibility that the difference of age and sex between two groups might affect our results.
Figures and Tables
Table 2
Echocardiographic variables after pacemaker implantation

*p < 0.05 versus apical pacing, †p < 0.05 versus the control. SPWMD: septal to posterior wall motion delay, S': systolic annular tissue velocity, Sm: averaged value of the peak systolic tissue velocity in the 6 basal segments, Ts: time to the peak systolic velocity with reference to the QRS complex, Tε: time to the peak strain with reference to the QRS complex, SL: time difference between the basal septum and lateral segment, SD: standard deviation of the time difference in 12 basal and mid segments
Acknowledgements
This work was supported by a research grant from the Korean Society of Echocardiography 2007.
References
1. Daggett WM, Bianco JA, Powell WJ Jr, Austen WG. Relative contributions of the atrial systoleventricular systole interval and of patterns of ventricular activation to ventricular function during electrical pacing of the dog heart. Circ Res. 1970. 27:69–79.

2. Tops LF, Schalij MJ, Holman ER, van Erven L, van der Wall EE, Bax JJ. Right ventricular pacing can induce ventricular dyssynchrony in patients with atrial fibrillation after atrioventricular node ablation. J Am Coll Cardiol. 2006. 48:1642–1648.

3. Manolis AS. The deleterious consequences of right ventricular apical pacing: time to seek alternate site pacing. Pacing Clin Electrophysiol. 2006. 29:298–315.

4. Padeletti L, Lieberman R, Schreuder J, Michelucci A, Collella A, Pieragnoli P, Ricciardi G, Eastman W, Valsecchi S, Hettrick DA. Acute effects of His bundle pacing versus left ventricular and right ventricular pacing on left ventricular function. Am J Cardiol. 2007. 100:1556–1560.

5. Kypta A, Steinwender C, Kammler J, Leisch F, Hofmann R. Long-term outcomes in patients with atrioventricular block undergoing septal ventricular lead implantation compared with standard apical pacing. Europace. 2008. 10:574–579.

6. Gorcsan J III. Role of echocardiography to determine candidacy for cardiac resynchronization therapy. Curr Opin Cardiol. 2008. 23:16–22.

7. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005. 352:1539–1549.

8. Pitzalis MV, Iacoviello M, Romito R, Massari F, Rizzon B, Luzzi G, Guida P, Andriani A, Mastropasqua F, Rizzon P. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol. 2002. 40:1615–1622.

9. Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004. 44:1834–1840.

10. Yu CM, Fung JW, Zhang Q, Chan CK, Chan YS, Lin H, Kum LC, Kong SL, Zhang Y, Sanderson JE. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation. 2004. 110:66–73.

11. Miyazaki C, Powell BD, Bruce CJ, Espinosa RE, Redfield MM, Miller FA, Hayes DL, Cha YM, Oh JK. Comparison of echocardiographic dyssynchrony assessment by tissue velocity and strain imaging in subjects with or without systolic dysfunction and with or without left bundle-branch block. Circulation. 2008. 117:2617–2625.

12. Kjaergaard J, Hassager C, Oh JK, Kristensen JH, Berning J, Sogaard P. Measurement of cardiac time intervals by Doppler tissue M-mode imaging of the anterior mitral leaflet. J Am Soc Echocardiogr. 2005. 18:1058–1065.

13. Suffoletto MS, Dohi K, Cannesson M, Saba S, Gorcsan J III. Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation. 2006. 113:960–968.

14. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003. 107:2932–2937.

15. Tse HF, Lau CP. Long-term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol. 1997. 29:744–749.

16. Thambo JB, Bordachar P, Garrigue S, Lafitte S, Sanders P, Reuter S, Girardot R, Crepin D, Reant P, Roudaut R, Jais P, Haissaguerre M, Clementy J, Jimenez M. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation. 2004. 110:3766–3772.

17. Doshi RN, Daoud EG, Fellows C, Turk K, Duran A, Hamdan MH, Pires LA. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J Cardiovasc Electrophysiol. 2005. 16:1160–1165.

18. Lieberman R, Padeletti L, Schreuder J, Jackson K, Michelucci A, Colella A, Eastman W, Valsecchi S, Hettrick DA. Ventricular pacing lead location alters systemic hemodynamics and left ventricular function in patients with and without reduced ejection fraction. J Am Coll Cardiol. 2006. 48:1634–1641.

19. Tse HF, Yu C, Wong KK, Tsang V, Leung YL, Ho WY, Lau CP. Functional abnormalities in patients with permanent right ventricular pacing: the effect of sites of electrical stimulation. J Am Coll Cardiol. 2002. 40:1451–1458.

20. Pastore G, Noventa F, Piovesana P, Cazzin R, Aggio S, Verlato R, Zanon F, Baracca E, Roncon L, Padeletti L, Barold SS. Left ventricular dyssynchrony resulting from right ventricular apical pacing: relevance of baseline assessment. Pacing Clin Electrophysiol. 2008. 31:1456–1462.

21. Tops LF, Suffoletto MS, Bleeker GB, Boersma E, van der Wall EE, Gorcsan J 3rd, Schalij MJ, Bax JJ. Speckle-tracking radial strain reveals left ventricular dyssynchrony in patients with permanent right ventricular pacing. J Am Coll Cardiol. 2007. 50:1180–1188.

22. Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002. 288:3115–3123.

23. Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick D, Sheldon T, Lamas GA. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007. 357:1000–1008.

24. Albertsen AE, Nielsen JC, Poulsen SH, Mortensen PT, Pedersen AK, Hansen PS, Jensen HK, Egeblad H. DDD(R)-pacing, but not AAI(R)-pacing induces left ventricular desynchronization in patients with sick sinus syndrome: tissue-Doppler and 3D echocardiographic evaluation in a randomized controlled comparison. Europace. 2008. 10:127–133.

25. Flevari P, Leftheriotis D, Fountoulaki K, Panou F, Rigopoulos AG, Paraskevaidis I, Kremastinos DT. Long-term nonoutflow septal versus apical right ventricular pacing: relation to left ventricular dyssynchrony. Pacing Clin Electrophysiol. 2009. 32:354–362.

26. de Cock CC, Meyer A, Kamp O, Visser CA. Hemodynamic benefits of right ventricular outflow tract pacing: comparison with right ventricular apex pacing. Pacing Clin Electrophysiol. 1998. 21:536–541.

27. Giudici MC, Thornburg GA, Buck DL, Coyne EP, Walton MC, Paul DL, Sutton J. Comparison of right ventricular outflow tract and apical lead permanent pacing on cardiac output. Am J Cardiol. 1997. 79:209–212.

28. Victor F, Leclercq C, Mabo P, Pavin D, Deviller A, de Place C, Pezard P, Victor J, Daubert C. Optimal right ventricular pacing site in chronically implanted patients: a prospective randomized crossover comparison of apical and outflow tract pacing. J Am Coll Cardiol. 1999. 33:311–316.

29. Rosenqvist M, Bergfeldt L, Haga Y, Ryden J, Ryden L, Owall A. The effect of ventricular activation sequence on cardiac performance during pacing. Pacing Clin Electrophysiol. 1996. 19:1279–1286.

30. Occhetta E, Bortnik M, Magnani A, Francalacci G, Piccinino C, Plebani L, Marino P. Prevention of ventricular desynchronization by permanent para-Hisian pacing after atrioventricular node ablation in chronic atrial fibrillation: a crossover, blinded, randomized study versus apical right ventricular pacing. J Am Coll Cardiol. 2006. 47:1938–1945.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download