Abstract
Aortitis is the all-encompassing pathological term ascribed to inflammation of the aorta. Regardless of the etiology, it frequently results in aortic root dilatation and aortic insufficiency rather than aortic stenosis. The rare case of aortitis such as isolated idiopathic aortitis may occur without evidence of systenic inflammatory disease or infection, and usually has subclinical nature. Even though the goals of therapy include immediate treatment of aortic inflammation or infection, the optimal management of isolated idiopathic aortitis is uncertain. We report a rare case of isolated idiopathic aortitis mimicking acute severe aortic stenosis, which was improved after steroid therapy.
Aortitis is the general name for a spectrum of disorders involving inflammation of the aorta. The cases of aortitis are classified to infectious and non-infectious diseases (inflammatory). The most common causes of non-infectious aortitis are the large-vessel vasculitis, such as giant cell arteritis (GCA) and Takayasu's arteritis. In addition to these arterities, other inflammatory disorders include rheumatoid arthritis, ankylosing spondylitis, Wegener's arteritis, and Behcet's disease.1) The underlying causes of aortitis and its concomitant manifestations determine the presenting symptoms. Aortitis, regardless of the etiology, frequently results in dilatation of the aortic root and aortic insufficiency rather than aortic stenosis. Isolated idiopathic aortitis which presented with severe aortic stenosis is extremely rare and has not been reported in Korea yet. In this case report, we present a rare case of isolated idiopathic aortitis mimicking acute severe aortic stenosis, which was improved after steroid therapy.
A 53-year-old man was admitted to the hospital with dizziness and New York Heart Association class II exertional dyspnea for 3 days. There was no remarkable familial history. He had received an implantable cardioverter-defibrillator due to idiopathic ventricular tachycardia (VT) 3 months ago and took antiarrhythmic medications. Initial 12-lead electrocardiogram (ECG) showed complete atrioventricular block and artificially paced rhythm of 40 beats per minute. The physical examination revealed newly developed systolic murmur of grade 4/6 at the right and left sternal borders. At first, we checked transthorasic echocardiogram (TTE) and it demonstrated severe aortic stenosis and mild aortic regurgitation due to swollen aortic leaflets (Fig. 1) with normal left ventricular systolic function. Aortic valve area was measured as 1.0 cm2 and thickness of aortic leaflets was about 12 mm (Fig. 1A and B). Peak and mean pressure gradients through aortic valve were elevated up to 79 and 48 mmHg, respectively (Fig. 1C). Initial white blood cell count was 11,200/mL, hemoglobin was 10.7 g/dL, and platelet count was 370,000/mL. Serum levels of cardiac enzymes and hormonal study were normal, but N-terminal pro-B-type natriuretic peptide (NT-proBNP) was elevated at 1,870 pg/mL, and serum levels of high sensitive C-reactive protein (hs-CRP) and erythrocyte sedimentation rate (ESR) were increased at 13.25 mg/dL and 112 mm/hr, respectively. On the next day, transesophageal echocardiography was performed and it revealed thickened aortic root wall, anterior mitral leaflet, and left atrial wall (Fig. 2). Their thickness was 9.3 mm, 7.2 mm, and 6.3 mm, respectively. Three month ago, TTE showed no significant abnormality of aortic valve and root. At that time, all of his laboratory findings were normal except ESR of 41 mm/hr, hs-CRP of 1.74 mg/dL and serum hemoglobin of 11.9 mg/dL. Cardiac biopsy and magnetic resonance imaging (MRI) showed no abnormalities. So, he has been diagnosed as idiopathic VT.
This time, there was no evidence of systemic infection including negative results on blood and urine cultures. The screening tests of human immunodeficiency virus and syphilis were negative. We could exclude acute rheumatic disease according to modified Jones criteria. All of the serologic markers for autoimmune diseases were normal and his pathergy test showed was negative result. These laboratory results were consistent with those of prior admission. We performed positron emission tomography (PET) scan and it showed increased uptake of fluorine-18-fluorodeoxyglucose (F-18-FDG) at the aortic arch but significant F-18-FDG uptake was not shown at the aortic valve probably because of lack of resolution to detect highly moving structure (Fig. 3). The results of biopsy at right temporal artery were unremarkable. We started steroid pulse therapy with prednisolone 60 mg/day under the diagnosis of idiopathic aortitis. Three days later, his symptoms were markedly improved and ECG showed first degree AV block. Level of serum BNP became normalized, and hs-CRP and ESR decreased to 1.92 mg/dL and 32.4 mm/hr, respectively. In follow up TTE, swelling of aortic valve leaflet and aortic root were not completely resolved but markedly improved (Fig. 4). Aortic valve area was increased to 3.12 cm2, and the thickness of aortic leaflet and aortic root wall was decreased as 5.3 mm and 4.2 mm, respectively (Fig. 4A and B). Additionally, peak and mean pressure gradients of aortic valve were measured as 31.6 and 16.0 mmHg, respectively (Fig. 4C). The patient discharged without symptoms and followed up with prednisolone of 10 mg/day.
Aortitis is the histopathological term for inflammation of the aortic wall. The classification of aortitis broadly includes systemic autoimmune and infectious diseases, along with isolated idiopathic aortitis.2) The category of aortitis is nonspecific granulomatous or isolated idiopathic aortitis, which may occur in patients without underlying systemic vasculitis or autoimmune syndrome.3) In the present case, although a tissue biopsy of the aorta has not been performed to confirm the diagnosis of the aortitis, we could exclude other types of aortitis including autoimmune aortitis, Takayasu's arteritis, and GCA based on serologic tests, history of the patient, temporal artery biopsy, and MRI findings. Thus, it seems likely that the diagnosis of this case is "idiopathic aortitis".
The presenting symptoms of aortitis were determined by the underlying cause of the aortitis and its concomitant manifestations. Therefore, the symptoms of aortitis vary with ranging from back or abdominal pain to acute severe aortic insufficiency or incidentally identified large thoracic aortic aneurysm.3) In the case of idiopathic isolated aortitis of the thoracic aorta, the inflammation of aorta are subclinical in nature and usually are diagnosed incidentally at the time of histopathology review after thoracic aortic aneurysm surgery.4) In the review of literature, however, we could not find any case of isolated idiopathic aortitis presenting with acute severe aortic stenosis. Regardless of the extent of aortitis, the most findings in several studies showing isolated valvulitis or aortitis were the aortic insufficiency and aneurysmal change of aortic root.5)6) To the best of our knowledge, this is the first case of isolated idiopathic aortitis presenting with acute severe aortic stenosis.
The diagnostic imaging modalities include computed tomography (CT), MRI, PET and echocardiography. Transthoracic or transesophageal echocardiography may demonstrate circumferential thickening of the aortic wall. Also, it plays a key role in the evaluation of the aortic root and valve in aortitis involving the ascending aorta associated with aortic insufficiency and aneurysm formation.7)8) In our case, echocardiography elucidated the morphological and hemodynamic changes of aortic valve, and it is clearly useful in monitoring of response of steroid therapy. Recently, the use of F-18-FDG PET, either alone or in combination with contrast-enhanced CT or MRI, has emerged as a potential tool for the initial diagnosis and assessment of disease activity of aortitis caused by either GCA or Takayasu's arteritis.9)10) Although PET scan showed increased uptake of F-18-FDG at thoracic aorta, we could not confirm the specific vasculitis because the results of histologic and immunologic data did not compatible to those diseases. Also, it did not show significant F-18-FDG uptake at aortic valve but this finding could not exclude the involvement of aortic valve because PET scan may often have limitations on some rapidly moving structures such as aortic valve.
The therapy of aortitis was focused on the immediate treatment of aortic inflammation, infection, and reduction of its complications. In noninfectious aortitis, immunosuppressive agents including steroid or methotrexate are the primary treatment but the treatment of corticosteroid in isolated idiopathic arteritis is controversial.3)8) Because the clinical outcomes of isolated idiopathic aortitis have been demonstrated only in small series of patients, the application of steroid therapy should be considered on a case-by-case basis, depending on the clinical presentation of the patient and the location and extent of inflammation.3) In the present report, steroid therapy was decided carefully and rapidly before moving to surgical intervention because symptoms of the patient were progressive in spite of other medical supports.
In this report, we present a rare case of isolated idiopathic aortitis mimicking severe aortic stenosis. The patient was successfully treated with steroid therapy. Echocardiography was useful not only for assessment of hemodynamic changes resulting from aortitis but for monitoring of response to steroid therapy.
Figures and Tables
Fig. 1
Parasternal long axis (A) and short axis views (B) reveal swollen aortic valve (AV) leaflets (arrow) and markedly narrowed AV area. These morphological changes result in severe stenotic physiology of AV with a mean transvalvular gradient of 48 mmHg and peak transvalvular aortic pressure of 79 mmHg (C). LV: left ventricle, LA: left atrium, RV: right ventricle, RA: right atrium.
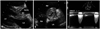
Fig. 2
Aortic valve (AV) long axis view demonstrates severe swelling of aortic root (arrows) and periaortic apparatus more clearly (A). AV short axis view shows narrowed AV orifice (B). LV: left ventricle, LA: left atrium, RV: right ventricle, RA: right atrium.
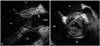
Fig. 3
Both of coronal (A) and sagittal views (B) show increased uptake of fluorine-18-fluorodeoxyglucose in the aortic arch (arrow head), but not in the aortic valve (arrow). LV: left ventricle.
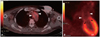
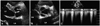
Fig. 4
In follow up transthorasic echocardiography, parasternal long axis (A) and short axis views (B) demonstrate decreased thickness of aortic leaflet and aortic root wall. Also, peak and mean pressure gradients of aortic valve are improved and measured as 31.6 and 16.0 mmHg, respectively (C). LV: left ventricle, LA: left atrium, RA: right atrium.
References
1. Tavora F, Burke A. Review of isolated ascending aortitis: differential diagnosis, including syphilitic, Takayasu's and giant cell aortitis. Pathology. 2006. 38:302–308.

2. Foote E, Postier RG, Greenfield RA, Bronze MS. Infectious aortitis. Curr Treat Options Cardiovasc Med. 2005. 7:89–97.

4. Rojo-Leyva F, Ratliff NB, Cosgrove DM 3rd, Hoffman GS. Study of 52 patients with idiopathic aortitis from a cohort of 1,204 surgical cases. Arthritis Rheum. 2000. 43:901–907.

5. Poppas A, Coady M. Echocardiographic findings and cardiac surgical implications of aortitis and valvulitis in Behçet's Disease. J Am Soc Echocardiogr. 2009. 22:1275–1278.

6. Beg A, Sadiq M. Subclinical valvulitis in children with acute rheumatic Fever. Pediatr Cardiol. 2008. 29:619–623.

7. Soto ME, Espinola-Zavaleta N, Ramirez-Quito O, Reyes PA. Echocardiographic follow-up of patients with Takayasu's arteritis: five-year survival. Echocardiography. 2006. 23:353–360.

8. Tsui KL, Lee KW, Chan WK, Chan HK, Hon SF, Leung TC, Lee KL, Tsoi TH, Li SK. Behçet's aortitis and aortic regurgitation: a report of two cases. J Am Soc Echocardiogr. 2004. 17:83–86.

9. Kobayashi Y, Ishii K, Oda K, Nariai T, Tanaka Y, Ishiwata K, Numano F. Aortic wall inflammation due to Takayasu arteritis imaged with 18F-FDG PET coregistered with enhanced CT. J Nucl Med. 2005. 46:917–922.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download