1. Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007. 153:907–917.

2. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002. 288:2709–2716.

3. Eckel RH, Barouch WW, Ershow AG. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on the pathophysiology of obesity-associated cardiovascular disease. Circulation. 2002. 105:2923–2928.

4. Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008. 94:e7.

5. Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003. 88:5163–5168.

6. Krysiak R, Labuzek K, Okopień B. Effect of atorvastatin and fenofibric acid on adipokine release from visceral and subcutaneous adipose tissue of patients with mixed dyslipidemia and normolipidemic subjects. Pharmacol Rep. 2009. 61:1134–1145.

7. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986. 1:307–310.

8. Ho E, Shimada Y. Formation of the epicardium studied with the scanning electron microscope. Dev Biol. 1978. 66:579–585.

9. Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, Masuda Y. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001. 157:203–209.

10. Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B. 1989. 94:225–232.

11. Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes. 1990. 14:1013–1022.
12. Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005. 2:536–543.

13. Iacobellis G, Pond CM, Sharma AM. Different "weight" of cardiac and general adiposity in predicting left ventricle morphology. Obesity (Silver Spring). 2006. 14:1679–1684.

14. Iacobellis G, Sharma AM. Adiposity of the heart. Ann Intern Med. 2006. 145:554–555.

15. Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, Leonetti F. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003. 11:304–310.

16. Park EM, Choi JH, Shin IS, Yun KH, Yoo NJ, Oh SK, Kim NH, Jeong JW. Echocardiographic epicardial fat thickness on short term prognosis in patients with acute coronary syndrome. J Cardiovasc Ultrasound. 2008. 16:42–47.

17. Iacobellis G, Pellicelli AM, Sharma AM, Grisorio B, Barbarini G, Barbaro G. Relation of subepicardial adipose tissue to carotid intima-media thickness in patients with human immunodeficiency virus. Am J Cardiol. 2007. 99:1470–1472.

18. Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK, Rhee SJ, Lee EM, Lee J, Yoo NJ, Kim NH, Park JC. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007. 71:536–539.

19. Okauchi Y, Nishizawa H, Funahashi T, Ogawa T, Noguchi M, Ryo M, Kihara S, Iwahashi H, Yamagata K, Nakamura T, Shimomura I, Matsuzawa Y. Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care. 2007. 30:2392–2394.

20. Busetto L, Tregnaghi A, Bussolotto M, Sergi G, Benincà P, Ceccon A, Giantin V, Fiore D, Enzi G. Visceral fat loss evaluated by total body magnetic resonance imaging in obese women operated with laparascopic adjustable silicone gastric banding. Int J Obes Relat Metab Disord. 2000. 24:60–69.

21. Iacobellis G, Sharma AM. Epicardial adipose tissue as new cardio-metabolic risk marker and potential therapeutic target in the metabolic syndrome. Curr Pharm Des. 2007. 13:2180–2184.

22. Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring). 2008. 16:1693–1697.

23. Steinberg D. The statins in preventive cardiology. N Engl J Med. 2008. 359:1426–1427.

24. Ray KK, Cannon CP. The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol. 2005. 46:1425–1433.

25. Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006. 5:1.

26. Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet. 2001. 357:577–581.

27. Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E. ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008. 358:1431–1443.

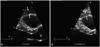
 . The intraobserver agreement was 93.8% and the COV was 3.2% (p < 0.001). The interobserver agreement was 93.2% and the COV was 4.6% (p < 0.001).
. The intraobserver agreement was 93.8% and the COV was 3.2% (p < 0.001). The interobserver agreement was 93.2% and the COV was 4.6% (p < 0.001). . The intraobserver agreement was 93.8% and the COV was 3.2% (p < 0.001). The interobserver agreement was 93.2% and the COV was 4.6% (p < 0.001).
. The intraobserver agreement was 93.8% and the COV was 3.2% (p < 0.001). The interobserver agreement was 93.2% and the COV was 4.6% (p < 0.001).