Abstract
We report here on 2 cases of idiopathic left atrial appendage ostial stenosis (LAA), and this rare finding was detected on transesophageal echocardiography. Its clinical implication is still unknown, given the small number of reported cases. Incompletely ligated LAA has characteristics similar to those observed in idiopathic LAA ostial stenosis, including the narrowed orifice, the small LAA cavity and the accelerated blood flow across the stenotic area. Since the incompletely ligated LAA has been reported to be complicated with thromboembolic events, we can assumed that the patients with idiopathic LAA ostial stenosis have a higher risk of thromboembolism than those with a normal LAA structure.
The left atrial appendage (LAA) is known as a small, muscular extension of the left atrium. Its clinical importance is that the LAA is a place where thrombus could be formed when the left atrial function decreases. In a study on 500 autopsy cases, the size of the LAA orifice is increased in the first 20 years of human life.1) The size of the LAA orifice reaches approximately 11 mm (6 mm to 20 mm) in men and 10 mm (5 mm to 18 mm) in women after the growth phase. Here we report on 2 cases of LAA that had a narrowed orifice and this was all incidentally detected on the transesophageal echocardiography.
A 70-year-old woman with a history of hypertension presented to our outpatient department complaining of recently developed exertional dyspnea. On physical examination, her blood pressure was 100/70 mmHg and the pulse rate was 105/min with an irregular rhythm. Auscultation of the lungs revealed bilateral crackles at both lower lung fields, and a faint systolic murmur was noted at the mitral valve area. The electrocardiography showed atrial fibrillation with a rate of 100 beats per minute.
A transthoracic echocardiogram revealed diffusely hypokinetic left ventricular wall motion with an ejection fraction of 35-40% and mild aortic valve regurgitation. The left atrium was enlarged and its diameter was 42 mm. Transesophageal echocardiography was conducted to identify the presence of thrombus before performing electrical cardioversion. This study revealed marked spontaneous echo contrast in the left atrium without any visible thrombus. The LAA had a small tubular shape, and the orifice was narrow with a diameter of 4.8 mm (Fig. 1A). Color turbulence across the orifice of the LAA with a peak velocity of more than 100 cm/sec was also noted (Fig. 1B and C). The direct current external cardioversion was performed without any complication and the patient was discharged with maintaining normal sinus rhythm.
A 68-year-old male presented with newly developed palpitation. He had a history of diabetes mellitus, hypertension and coronary artery disease along with a history of coronary artery bypass surgery 16 years ago.
On physical examination, his blood pressure was 120/70 mmHg and the heart rate was approximately 140/min. Auscultation of the lung and heart was not remarkable. The electrocardiography showed atrial flutter with 2 : 1 ventricular conduction. Pharmacological cardioversion was tried first, but this failed.
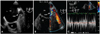
A transthoracic echocardiogram revealed global left ventricular systolic dysfunction with an ejection fraction of 35% and a dilated left atrium. On the transesophageal echocardiography, neither intracardiac thrombus nor spontaneous echo contrast was seen. The diameter of the orifice of the LAA was 3.6 mm, and the body of the LAA showed a long tubular shape (Fig. 2A). The flow was accelerated at the ostium of the LAA with peak velocities of more than 110 cm/sec (Fig. 2B and C). Direct current external cardioversion was successfully performed, and the patient was discharged without any adverse events.
The LAA ostial stenosis is a very rare finding that is generally detected incidentally on transesophageal echocardiography. LAA ostial stenosis can be classified into 2 categories: one is the LAA with a congenitally narrowed orifice and the other is a remnant LAA after incomplete LAA ligation, which is conducted during open cardiac surgery. According to a previous report that analyzed 500 autopsy cases, the size of the normal LAA orifice ranges from 6 mm to 20 mm in men and from 5 mm to 18 mm in women. Thus, the size of a LAA orifice less than 5 mm could be sufficient for the diagnosis of LAA ostial stenosis.1) In our cases, the LAA orifice measured between 3.8 to 4.8 mm, and significant flow acceleration across the orifice was observed as well.
Our patient of the second case had a history of cardiac surgery 16 years ago. The patient's operation record was not found, so it was not clear whether the narrowed orifice of the LAA was idiopathic LAA ostial stenosis or a postoperative complication. However, the patient's electrocardiography showed normal sinus rhythm before the coronary artery bypass surgery, and the preoperative transthoracic echocardiography revealed the normal structure of the mitral valve, and there was no significant enlargement of the left atrium. On discharge summary note, only 1 operation name was written. In addition, the LAA exclusion operation was not routinely performed in our hospital at that time unless the patient was on the Maze operation. Thus, it is plausible to consider our patient's findings as idiopathic LAA ostial stenosis rather than an incompletely ligated LAA. Furthermore, the transesophageal echocardiographic findings were similar for both patients.
Since there have been few case reports of this malady, the incidence, pathophysiology and clinical implications of idiopathic LAA ostial stenosis are unclear. In a previous report, the possibility was suggested that the relative blood stagnation behind the stenotic area could increase the risk of thrombus formation in the LAA.2) In another report, the accelerated flow across the stenotic area was assumed to have injured the endocardial tissue and this resulted in endocarditis.3) However, these reports failed to show direct associations with the patients' clinical events.
On the other hand, there have been a relatively larger number of cases on LAA ostial stenosis after incomplete surgical ligation. An incompletely ligated LAA is known to have similar echocardiographic findings as those observed for idiopathic LAA ostial stenosis, including the LAA morphology, the narrowed LAA orifice and the accelerated blood flow across the stenotic area. The various clinical manifestations of the incompletely ligated LAA have been reported, including thrombus formation in the LAA and the patient's thromboembolic events.
Rosenzweig et al.4) reported on a case of a 69-year-old man with a history of mitral valve repair 4 years previously and a recent cerebrovascular event; the transesophageal echocardiography revealed an incompletely ligated LAA and thrombus within it. As no other cause of stroke was found, the authors thought that the LAA thrombus was associated with the patient's cerebrovascular event. There were 2 reported cases of incompletely ligated LAA, in which thrombus formation occurred during the postoperative period.5) In a study analyzing 50 patients who underwent mitral valve surgery and ligation of the LAA, they reported that the patients with incomplete ligation had increased incidence of thromboembolic events.6) They found that 18 of 50 patients had incompletely ligated LAA, and 4 of 18 patients (22%) had thromboembolic events. In the study that focused on 137 patients with a previous history of LAA closure, only 55 of 137 (40%) closure were successful, and transesophageal echocardiography revealed LAA thrombus in 28 of 68 patients (41%) with unsuccessful closure.7) They found that 12 patient with unsuccessful closure (15%) had evidence of stroke or transient ischemic attack. There is still debate about the safety and usefulness of LAA exclusion surgery, yet there is agreement among authors that patients with a remnant LAA have a higher risk of thromboembolism.
As observed in the cases with an incompletely ligated LAA, the patients with idiopathic LAA ostial stenosis could be considered to have a higher risk of thromboembolic events than the patients with a normal LAA structure. Neither intracardiac thrombus formation nor significant cerebrovascular events were demonstrated in our cases, yet marked spontaneous echo contrast in the left atrium and LAA was observed in 1 case. The patient with spontaneous echo contrast was on aspirin, whereas the other patient was on anticoagulation treatment. In a study that analyzed patients with nonrheumatic atrial fibrillation, the incidence of spontaneous echo contrast and ischemic stroke was significantly lower in the subgroup with a high LAA flow profile (higher than 20 cm/sec).8) The finding that the spontaneous echo contrast exists despite the high LAA flow velocity and taking antiplatelet agent implies that anticoagulation therapy could be safer way to prevent thromboembolic events in a patient with idiopathic LAA ostial stenosis, and especially when LAA dysfunction occurs. More experience, further investigation and long term follow up data are all needed to clarify the clinical meaning of LAA ostial stenosis.
Figures and Tables
Fig. 1
Transesophageal echocardiography of the case 1 revealed a small tubular shaped left atrial appendage with a narrowed orifice, and the maximal diameter of the orifice was only 4.8 mm (A). Doppler examination showed significant flow acceleration across the stenotic area with a peak velocity more than 100 cm/sec (B and C). LA: left atrium, LAA: left atrial appendage, LV: left ventricle.
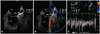
Fig. 2
Transesophageal echocardiography of the case 2 revealed a long tubular shaped left atrial appendage with a narrowed orifice, and the maximal diameter of the orifice was only 3.8 mm (A). The peak velocity across the narrowed orifice measured more than 110 cm/sec with flow acceleration on Doppler echocardiography (B and C). LA: left atrium, LAA: left atrial appendage, LV: left ventricle.
References
1. Veinot JP, Harrity PJ, Gentile F, Khandheria BK, Bailey KR, Eickholt JT, Seward JB, Tajik AJ, Edwards WD. Anatomy of the normal left atrial appendage: a quantitative study of age related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997. 96:3112–3115.
2. Coughlan B, Lang RM, Spencer KT. Left atrial appendage stenosis. J Am Soc Echocardiogr. 1999. 12:882–883.

3. Stern JD, Skolnick AH, Freedberg RS, Kronzon I. Isolated left atrial appendage ostial stenosis. Eur J Echocardiogr. 2009. 10:702–703.

4. Rosenzweig BP, Katz E, Kort S, Schloss M, Kronzon I. Thromboembolus from a ligated left atrial appendage. J Am Soc Echocardiogr. 2001. 14:396–398.

5. Oneglia C, Muneretto C, Rusconi C. Transesophageal investigation of surgically ligated left atrial appendage. Echocardiography. 2004. 21:617–619.

6. Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiographic study. J Am Coll Cardiol. 2000. 36:468–471.

7. Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008. 52:924–929.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download