Abstract
Cardiac papillary fibroelastoma (CPF) is a benign cardiac tumor that usually affects cardiac valves. It is usually discovered incidentally on routine echocardiography. However, left ventricular CPF is rare. This report describes the case of a 73-year-old female, referred to a cardiology department for evaluation of a mass of the left ventricle. The mass was found routine echocardiography. The transthoracic echocardiography revealed a 2.2×1.3 cm highly oscillating mass, attached by stalk on the inferior wall of the left ventricle. Cardiac magnetic resonance imaging demonstrated a non-enhanced, 1.8×1.0 cm mass on the inferior wall of the left ventricle. The patient underwent surgical resection of the mass, histopathologic examination of the mass confirmed the diagnosis of a CPF.
According to large autopsy series, primary cardiac tumors are very rare, with a reported frequency of 0.02%.1) They are usually discovered incidentally on routine echocardiography (TTE). Cardiac papillary fibroelastoma (CPF) is a benign cardiac tumor with potential for causing life-threatening embolic events. CPF is the third most common primary tumor of the heart and most commonly affects cardiac valves.2) Surgical excision of the tumor is recommended for all patients who develop symptoms. In particular, for asymptomatic left-sided, mobile CPF which could flow in systemic circulation, surgical resection is recommended.3)4) We report on a case of left ventricular CPF discovered on echocardiography, and removed by surgical resection.
A 73-year-old female with past medical history of diabetes, rheumatoid arthritis, and chronic renal insufficiency was referred to our cardiology department for evaluation of a cardiac mass of left ventricle which is incidentally found on a routine TTE. The patient had no related symptoms. Physical exam and routine laboratory, electrocardiogram, chest X-ray, and laboratory data were unremarkable, except for mildly elevated serum creatinine level. On the TTE examination, the left ventriclular ejection fraction was normal and mild mitral regurgitation was found. TTE also revealed a 2.2×1.3 cm sized oval shaped highly oscillating oval shaped mass attached by stalk on the inferior wall of the left ventricle (Fig. 1). Transesophageal echocardiography revealed an oval shaped 1.7×1.0 cm sized echogenic mass attached by stalk on the inferior wall of left ventricle with area of echolucency (Fig. 2). Computer tomography (CT) of the chest showed mass like focal thickening of the ventricular septal wall. Cardiac magnetic resonance imaging demonstrated a non-enhanced, 1.8×1.0 cm mass on the inferior wall of the left ventricle (Fig. 3). The patient was referred to the department of thoracic surgery, turned out CPF on histopathologic examination (Fig. 4). After surgical resection, the patients was treated for pneumonia. Despite of adequate management, pneumonia deteriorated through long term use of steroid, diabetes and chronic renal insufficiency. At last, the patient expired by septic shock.
After myxoma and lipoma, CPF is the third most common primary tumor of the heart and most commonly affects cardiac valves.2) CPF clearly predominates in adults and is particularly frequent between the 4th and 8th decades of life. The male sex is predominant in most series. Most cases are probably acquired, however the etiology is unknown.3) Tumors consist of avascular papillomas covered by a single layer of endothelium. Grossly, CPF has a flower-like appearance with multiple papillary fronds attached to the endocardium by a short pedicle. Immersion in water after resection shows a typical sea anemone-like appearance. It provide easy morphological verification of the tumor as a CPF.3)
CPFs usually develop in cardiac valves. More than 95% arise in the left heart. The aortic valve is the most frequently involved followed by the mitral valve. Less frequent sites of involvement include the following: mitral chordae, right atrial endocardium, and endocardial surface of both ventricles including the papillary muscles and ventricular septum. The left ventricle is the most common non-valvular site of involvement.5) Tumor size varies, with 83% less than 1 cm in diameter.6)
Patients with CPF are generally asymptomatic, and tumors are noted as incidental findings on autopsy or echocardiography.3)7) However, because the tumor may induce life-threatening complications, diagnosis is important. Symptoms are induced by embolization, either of the thrombus or the tumor itself. The most common clinical presentations include stroke, acute coronary syndrome, heart failure, syncope, mesenteric ischemia, pulmonary embolism and sudden death.3) Clinical presentation is determined by location, size, and mobility of the tumor, systemic embolism is frequent in tumor arising from the left side of the heart.
Echocardiography usually demonstrates a small, mobile, pedunculated or sessile, valvular or endocardial mass, with fluttering in the cardiac chambers during systole or diastole. CPF may appear speckled with echolucencies and a stippled pattern near the edges, which correlates with papillary projections on the surface of the mass. Magnetic resonance imaging typically demonstrates a CPF on a valve leaflet or on the endocardial surface of the affected cardiac chamber.3)
CPFs are different from other cardiac tumors. Fibromas usually occur in children and young adults and typically involve the left ventricle, right ventricle and septum.8) Myxoma is developed predominantly at left atrial tumor. Histologically, myxoma shows blood vessels within papillae and polygonal myxoma cells.9) Cardiac lipoma can occur in any location and is well-encapsulated tumor made up by mature fat cells.10)
There are no guidelines for management of CPF. No data exist for evaluation of the efficacy of anticoagulation or antiplatelet therapy for CPF, although it is supposed that deposition of thrombotic material on tumors may add to the risk of micro-embolization.11) However, with no definite contraindications to surgery, the only independent predictor of mortality or non-fatal embolization is mobility. Surgical excision is certainly recommended for symptomatic patients or patients with a highly mobile CPF with a stalk.3) Surgical intervention, which considered safe, without significant morbidity or mortality, was the first line treatment recommended for this patient. Asymptomatic non-mobile or right side CPF could be followed-up closely.3)12) However, these recommendations are not based on the randomized controlled data. For asymptomatic left-sided CPF which could flow in systemic circulation, in particular, surgical resection is recommended, because incidence of life-threatening complications is higher.3)6)
This case illustrates surgical management of a pedunculated left ventricular mass, diagnosed by echocardiography, which confirmed the diagnosis of a CPF. The present case highlights an atypical presentation of a CPF attached to the inferior wall of the left ventricle, without valvular involvement and large mass size.
Figures and Tables
Fig. 1
Transthoracic echocardiography reveals an oval shaped mass (2.2×1.3 cm) attached by stalk on the inferior wall of left ventricle (arrow).
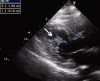
Fig. 2
Transesophageal echocardiography reveals an oval shaped echogenic mass (1.7×1.0 cm) attached by stalk on the inferior wall of left ventricle with area of echo-lucency (arrow).
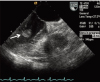
Fig. 3
Cardiac magnetic resonance imaging shows a non-enhanced, 1.8×1.0 cm mass with a stalk on the inferior wall of the left ventricle (arrow).
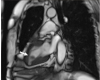
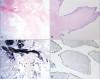
Fig. 4
Microscopic images show narrow, elongated and branching papillary fronds (H&E stain ×12.5) (A). Central avascular collagen and variable elastic tissue surrounded by acid mucopolysaccharide and lined by hyperplastic endothelial cells (H&E stain ×100) (B). Elastic fiber staining shows black colored core of papilla (elastc fiber stain ×12.5) (C). CD34 (endothelial cell marker) staining shows endothelial cell stained with brown color (CD34 stain ×200) (D).
References
2. Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg. 1991. 52:1127–1131.

3. Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003. 146:404–410.

4. Mutlu H, Demir IE, Leppo J, Levy WK. Nonsurgical management of a left ventricular pedunculated papillary fibroelastoma: a case report. J Am Soc Echocardiogr. 2008. 21:877.e4. 877.e7.

5. Moustafa S, Sauvé C, Pagé P, Serri K. Incidental finding of a papillary fibroelastoma of the mitral valve chordae. Eur J Echocardiog. 2008. 9:745–746.

6. al-Mohammad A, Pambakian H, Young C. Fibroelastoma: case report and review of the literature. Heart. 1998. 79:301–304.

7. Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol. 1997. 30:784–790.

8. Parmley LF, Salley RK, Williams JP, Head GB 3rd. The clinical spectrum of cardiac fibroma with diagnostic and surgical considerations: noninvasive imaging enhances management. Ann Thorac Surg. 1988. 45:455–465.

9. Burke A, Virmani R. Tumors of the heart and great vessels. Atlas of tumor pathology 3rd Series. 1996. 1st ed. Washington DC, USA: American Registry of Pathology;47–54.
10. Hong G, Byun YS, Kang S, Rim SJ, Chung N, Cho SY, Kim SS. A case of cardiac lipoma. J Korean Soc Echocardiogr. 2002. 10:8–10.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download