Abstract
Stress-induced cardiomyopathy is frequently confused with acute coronary syndromes. We encountered a 64-year old female patient with panhypopituitarism initially suspected as atypical stress-induced cardiomyopathy due to her history and initial echocardiographic findings. She was finally diagnosed as non ST-segment elevation myocardial infarction based on the findings of coronary angiogram, intravascular ultrasound and subsequent echocardiogram.
Stress-induced cardiomyopathy (SICM) is a disease characterized by abnormalities of electrocardiogram, transient left ventricular (LV) wall motion abnormality disproportionate to the area perfused by a single coronary artery, and normal coronary angiogram.1) The diagnosis of SICM can be frustrated with acute coronary syndrome when the patient presents without chest pain in stressful conditions.2) Here we report the case of a 64-year old female patient initially suspected as atypical SICM or "inverted Takotsubo" but finally diagnosed as acute coronary syndrome (ACS).
A 64-year old woman visited the emergency room due to decreased mentality. She had experienced general weakness since her last delivery. She complained of severe watery diarrhea, more than ten times a day, which had developed 2 days before the visit. On initial presentation, she had a blood pressure of 50/30 mmHg and a pulse rate of 119 beats per minute. Physical examination showed anemic conjunctivae and non-pitting pretibial edema. Her axillary and pubic hairs were absent. Her initial electrocardiogram (ECG) demonstrated T wave inversion II, III, aVF and chest leads (Fig. 1A). Complete blood count showed anemia and thrombocytopenia. (hemoglobin: 10.1 g/dL, platelet count: 130,000/mm2) Blood chemistry revealed significantly elevated levels of muscle enzymes (LDH/CPK 6133/779 mg/dL) and mildly elevated cardiac enzymes (CK-MB/troponin-I 8.5/0.03 ng/mL). Hyponatremia and elevated levels of hepatic enzymes and BUN/Creatinine were also detected (Na/K/Cl; 129/3.8/98 mEq/L, AST/ALT; 110/35 U/L, BUN/Creatinine: 33.2/1.6 mg/dL). The echocardiogram revealed akinesia of mid and basal portions of the LV with hyperkinesia of the apex (Fig. 2A and B).
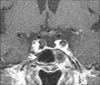
On the 2nd hospital day, cardiac enzymes peaked with troponin-I count of 29.78 ng/mL and CK-MB of 166 ng/mL. The inversions of T wave were normalized but the depressed ST segment remained (Fig. 1B). The condition of the patient was improved after administration of inotropics, hypertonic saline and low molecular weight heparin. The laboratory abnormalities, except elevated cardiac enzymes, were normalized by the 3rd hospital day. Cosyntropin stimulation test revealed adrenal insufficiency, and pituitary evaluation demonstrated central hypothyroidism and hypogonadism as well as hypoprolactinemia. Magnetic resonance imaging showed empty sella (Fig. 3). Predisolone and thyroxine were therefore prescribed.
Echocardiogram performed on the 5th hospital day demonstrated almost no interval change in the regional wall motion abnormalities of LV (Fig. 1C and D), and matching results were observed in the bull's eye display showing peak systolic longitudinal strain of the LV using automated functional imaging (Fig. 4A). Coronary angiography demonstrated significant stenosis of the left anterior descending artery (LAD) and mild stenosis of the right coronary artery (RCA). Quantitative coronary analysis of LAD showed 73% of diameter stenosis (reference diameter: 3.94 mm, minimal luminal diameter: 1.08 mm). Minimal luminal area calculated by intravascular ultrasound (IVUS) was 2.3 mm2. So percutaneous coronary intervention with a drug-eluting stent was performed on LAD (Fig. 5). IVUS exam was not performed on RCA. Echocardiogram performed 5 weeks after the initial visit showed akinesia of the basal inferior wall of LV remained although other abnormalities of regional wall motions were markedly improved (Fig. 2E, F and 4B).
SICM occurs most commonly in postmenopausal women in a setting of severe emotional and/or physical stress.3)4) The current diagnostic criteria require all of the following: 1) transient LV wall motion abnormalities, especially involving the apical and/or midventricular segments; 2) absence of obstructive coronary artery disease or angiographic evidence of acute plaque rupture; and 3) new ECG abnormalities or troponin elevation.5) Although apical ballooning is the typical finding of SICM, an atypical form that spared the apex of LV ("inverted" Takotsubo) has also been reported and this form was found in nearly 40% of SICM in a large consecutive study.6-8)
In this case, the patient visited the hospital due to decreased mentality but not chest pain; she was experiencing physical stress, e.g., panhypopituitarism and severe diarrhea, resulting in electrolyte imbalance and renal failure. Initial echocardiogram showed typical findings of atypical SICM. We therefore initially diagnosed her as atypical SICM although there was marked elevation of cardiac enzymes. Hypothyroidism accompanied by panhypopituitarism can induce elevated serum CPK levels and mimic acute coronary syndrome.9)10) In this patient, troponin-I was elevated upon initial presentation and peaked at more than 700 times the upper normal limit. These elevations are very unusual in hypothyroidism or SICM if the patient is not complicated by myocardial infarction.10)11) Regional LV function was not recovered 5 days after the initial presentation and there were also residual abnormalities on the echocardiogram performed 5 weeks later in this patient. In SICM, rapid normalization of regional LV function is common, although it can occur over the ensuing 1-3 months.5) According to the initial report, the coronary artery should be essentially normal to diagnose SICM; however, accompanying coronary lesions have recently been reported in many cases of SICM.12) So the diagnostic criteria also suggest not essentially normal epicardial artery but no obstructive lesion or evidence of acute plaque rupture.5) Coronary angiogram showed obstructive lesion of LAD. IVUS also demonstrated large burden of atheroma and decreased minimal luminal area of LAD resulting obstructive stenosis. Therefore, because of findings of coronary artery angiogram, IVUS, cardiac enzymes and serial echocardiogram, it would be more appropriate to diagnose ACS in this case, rather than atypical SICM.13)
Although we think that this patient should be diagnosed as ACS according to current diagnostic criteria, which includes absence of obstructive coronary artery disease or angiographic evidence of acute plaque rupture, regional cardiac function seemed to indicate atypical SICM on initial presentation. There's also possibility of although PCI was performed on LAD according to coronary angiography and IVUS findings, RCA was also involved such as coronary spasm or intracoronary thrombus, which were resolved spontaneously later
Finally, although typical history and echocardiogram may suggest SICM, this case demonstrates that cautious evaluation using coronary angiography, IVUS, serial echocardiogram and laboratory workup is essential to rule out ACS at the time of diagnosis.13)
Figures and Tables
Fig. 1
Electrocardiogram on admission (A) shows T wave inversion in II, III, aVF and chest leads. Follow-up electrocardiogram the next day (B) demonstrates normalization of T wave inversion, but ST segment depression remained in chest leads.
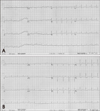
Fig. 2
Echocardiogram obtained upon admission shows basal dyskinesia with sparing of the apical wall motion on apical four-chamber view. (A: end diastole, B: end systole). Follow-up echocardiogram shows almost no interval change after 5 days. (C: end diastole, D: end systole). Echocardiogram obtained 5 weeks later demonstrates recovery of regional left ventricular function, except basal inferoseptal portion (E: end diastole, F: end systole).
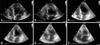
Fig. 4
A: Basal dyskinesia with sparing of the apical wall motion is demonstrated by bull's eye display showing peak systolic longitudinal strain of the left ventricle in automated functional imaging of follow-up echocardiogram performed on 5th hospital day. B: Dyskinesia remains, especially on basal inferoseptal wall of left ventricle, as is demonstrated by follow-up echocardiogram 5 weeks after initial presentation.
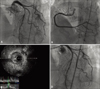
Fig. 5
A: Coronary angiogram shows significant stenosis of left anterior descending artery. B: Mild stenosis of right coronary artery is also observed. C: Intravascular ultrasound shows atheroma plaque resulting obstructive stenosis of LAD. D: Percutaneous coronary intervention is performed on the left anterior descending artery and there is no residual stenosis.
References
1. Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, Honda T, Hase M, Kai R, Morii I. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001. 38:11–18.

2. Nanda S, Pamula J, Bhatt SP, Chan I, Turki MA, Dale TH. Takotsubo cardiomyopathy--a new variant and widening disease spectrum. Int J Cardiol. 2007. 120:e34–e36.
3. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004. 141:858–865.

4. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006. 27:1523–1529.

6. Reuss CS, Lester SJ, Hurst RT, Askew JW, Nager P, Lusk J, Altemose GT, Tajik AJ. Isolated left ventricular basal ballooning phenotype of transient cardiomyopathy in young women. Am J Cardiol. 2007. 99:1451–1453.

7. Kurowski V, Kaiser A, von Hof K, Killermann DP, Mayer B, Hartmann F, Schunkert H, Radke PW. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest. 2007. 132:809–816.

8. Kim TS, Chu EH, Kang HH, Chun SW, Cho EJ, Kim JH. A case of reversal of takotsubo cardiomyopathy in patient with pheochromocytoma. J Cardiovasc Ultrasound. 2007. 15:50–54.

9. Goldman J, Matz R, Mortimer R, Freeman R. High elevations of creatine phosphokinase in hypothyroidism. An isoenzyme analysis. JAMA. 1977. 238:325–326.

10. Gunduz H, Arinc H, Yolcu M, Akdemir R, Kanat M, Uyan C. A case of hypothyroidism mimicking acute coronary syndrome. Int J Cardiovasc Imaging. 2006. 22:141–145.

11. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008. 155:408–417.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download