Abstract
Pedunculated thrombus in the aortic arch that is associated with cerebral infarction is very rare requires prompt diagnosis and treatment to prevent occurrence of another devastating complication. Transesophageal echocardiography is useful for detecting source of embolism including aortic thrombi. The treatment options of aortic thrombi involves anticoagulation, thrombolysis, thromboaspiration, and thrombectomy. Here we report a case of huge thrombus in the aortic arch, resulting in acute multifocal cerebellar embolic infarct in patient without any risk factors for vascular thrombosis. Thrombi in the aortic arch were diagnosed by transesophageal echocardiography and treated with anticoagulants successfully.
The heart is commonly suspected to be the major source of systemic emboli. More than 85% of major arterial emboli have their origin in heart. Emboli originating from protruding atheromas and aortic aneurysms are the main source of non-cardiac emboli. In recent years, non-cardiac sources of arterial embolism have been recognized with increasing frequency, whereas idiopathic causes have become less common.1) The incidence of arterial embolism of aortic origin has been reported in up to 5% of cases.2) However, pedunculated mobile thrombi in the aortic arch is a rare entity. We report a case of pedunculated thrombus, formed within localized atheromatous plaque in the aortic arch, leading to multifocal cerebellar infarct in a 41-year-old man without any known risk factor for vascular thrombosis.
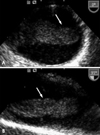
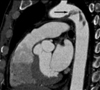
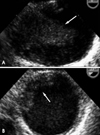
A 41-year-old man was to the neurology department in our institution with an acutely developed dizziness and dysarthria. He had a history of untreated dyslipidemia and was a heavy smoker. However, he had no medical history of arrhythmias, diabetes mellitus, ischemic heart disease or stroke. On admission, he was hemodynamically stable. During physical examination, ptosis and miosis on his right side were observed. Brain magnetic resonance imaging revealed acute infarct on the right posterior internal communicating artery territory and right cerebellum (Fig 1). Plain chest radiography and electrocardiogram were normal. Transthoracic echocardiography (TTE) that was perfomed to determine the source of embolism was unremarkable. Extensive serologic survey for hypercoagulable and vasculitis disorders (including antiphospholipid antibodies, antinuclear antibody, lupus anticoagulant, protein C/S) was all negative. Transesophageal echocardiography (TEE) was followed to exclude potential sources of embolism, revealing a 2.5×5 cm - sized huge protruding mobile mass attatched to broad atheromatous plaque in the aortic arch began at 25 cm from the incisors and extended to the descending thoracic aorta (Fig. 2). This mass was homogenous in consistency and adherent in part to the aortic wall. The agitated saline study during TEE for detecting patent foramen ovale was negative. The left atrium including left atrial appendage was unremarkable on TTE and TEE. To further evaluate the disease extent, multi-detector computed tomography (MDCT) was performed and also showed a large filling defect in the aortic arch (Fig. 3). The patient was treated with anticoagulation therapy immediately with intravenous unfractionated heparin (80 unit/kg bolus infusion, followed by 18 unit/kg/hr continuous infusion and adjusted in accordance with therapeutic range of activated partial thromboplastin time) and warfarin (overlap with heparin for 3 days initially, target international normalized ratio 2-3) for 14 days, and then follow-up TEE was performed. Follow-up TEE showed only small amount of remnant thrombus in atheromatous plaque of the aortic arch (Fig. 4). Over time, he progressively recovered neurologic deficits. Five days later, he was allowed to leave the hospital on anticoagulation therapy with warfarin. Further thromboembolic event has not occurred for 12 months of follow-up period.
Most cardiac source of embolism is caused by thrombi in the left side of the heart. However, aortic thrombi are another important cause of arterial thromboembolism.1) These aortic thrombi are frequently associated with some hypercoagulable states, e.g., antiphospholipid antibody syndrome, protein C/S deficiency and depressed activation of protein C.3)
Pedunculated thrombi in the thoracic aorta without any predisposing condition is very rare. These thrombi can move freely in the aortic lumen with each cardiac cycle, and their fragmentation can cause acute ischemic episodes due to cerebral, visceral, or peripheral arterial embolization.4)5) Pathologic studies of the aortic wall in these patients have shown lesions of atheroma, often minimal atherosclerotic plaques.6) These patients have established atherosclerotic lesions that can act as a nidus for thrombus formation that can deliver distal emboli. Mobile mural thrombus of the aortic arch differs distinctly from atheroembolism in pathogenesis, but pathogenesis of the aortic mural thrombus formation has not been clearly defined, yet being considered potentially multifactorial.7-9)
TEE has been reported to locate the source of arterial embolisms in patients with systemic arterial emboli more reliably than TTE could. High resolution images of the heart, aortic arch and the descending thoracic aorta can be obtained by TEE, which may provide useful information for locating aortic thrombus or aortic atherosclerotic conditions or for ruling out cardiac or aortic disease.10)11)
Therapeutic standard for these pedunculated lesions remains undetermined. Therapy for these patients should focus on preventing the evolution of these lesions. A variety of approaches are used, including conservative treatment with anticoagulation or thrombolysis, interventional modalities such as thromboaspiration, or balloon-catheter thrombectomy, and open surgical procedures such as thrombectomy, thromboendarterectomy, and aortic prosthetic replacement.12-16) Given currently available data from literature showing a high risk of recurrence, and a potentially high rate of operative mortality/morbidity of thoracotomy, medical therapy with anticoagulation is considered the first option for this rare, but potentially devastating disease.17)
In the present case, TEE allowed us to rule out other aortic diseases and raised the suspicion of aortic thrombus in young patient without any predisposing factor for vascular thrombosis, based on which anticoagulation therapy was initiated. Without surgical intervention, follow-up TEE confirmed it to be an aortic thrombus, suggesting again that TEE is accurate for diagnosing aortic mural thrombus. The long-term results of medical therapy for these aortic thrombi should be traced continuously, but embolization has not recurred during 12 months of follow-up period.
Figures and Tables
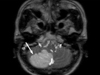
Fig. 1
Brain magnetic resonance imaging, acute infarct on right posterior internal communicating artery territory and multifocal cerebellum (arrow).
References
1. Panetta T, Thompson JE, Talkington CM, Garrett WV, Smith BL. Arterial embolectomy: a 34-year experience with 400 cases. Surg Clin North Am. 1986. 66:339–353.

2. Gagliardi JM, BattM , Khodja RH, Le bas P. Mural thrombus of the aorta. Ann Vasc Surg. 1988. 2:201–204.

3. Laperche T, Laurian C, Roudaut R, Steg PG. Mobile thromboses of the aortic arch without aortic debris. A transesophageal echocardiographic finding associated with unexplained arterial embolism. The Filiale Echocardiographie de la Société Française de Cardiologie. Circulation. 1997. 96:288–294.

4. Sadony V, Walz M, Löhr E, Rimpel J, Richter HJ. Unusual cause of recurrent arterial embolism: floating thrombus in the aortic arch surgically removed under hypothermic cardiocirculatory arrest. Eur J Car-diothorac Surg. 1988. 2:468–471.

5. Tunick PA, Kronzon I. Protruding atherosclerotic plaque in the aortic arch of patients with systemic embolization: a new finding seen by transesophageal echocardiography. Am Heart J. 1990. 120:658–660.

6. Rubin BG, Allen BT, Anderson CB, Barzilai B, Sicard GA. An embolizing lesion in a minimally diseased aorta. Surgery. 1992. 112:607–610.
7. Machleder HI, Takiff H, Lois JF, Holburt E. Aortic mural thrombus: an occult source of arterial thromboembolism. J Vasc Surg. 1986. 4:473–478.

8. Lozano P, Gomez FT, Julia J, M-Rimbau E, Garcia F. Recurrent embolism caused by floating thrombus in the thoracic aorta. Ann Vasc Surg. 1998. 12:609–611.

9. Song Jin-Ho, Park Geung-Dong, Cho Gil-Hyun, Kim Doo-Il, Kim Dong-Soo. Cerebral infarction caused by floating thoracic aortic throm-bus in young male. J Cardiovascular Ultrasound. 2000. 8:236–240.

10. Blanchard DG, Kimura BJ, Dittrich HC, DeMaria AN. Transesophageal echocardiography of the aorta. JAMA. 1994. 272:546–551.

11. Wiet SP, Pearce WH, McCarthy WJ, Joob AW, Yao JS, McPherson DD. Utility of transesophageal echocardiography in the diagnosis of disease of the thoracic aorta. J Vasc Surg. 1994. 20:613–620.

12. Blackshear JL, Jahangir A, Oldenburg WA, Safford RE. Digital embolization from plaque-related thrombus in the thoracic aorta: identification with transesophageal echocardiography and resolution with warfarin therapy. Mayo Clin Proc. 1993. 68:268–272.

13. Culliford At, Tunick PA, Katz ES, Kronzon I, Galloway AC, Ribakove GH, Esposito RA, Spencer FC. Initial experience with removal of protruding atheroma from the aortic arch: diagnosis by transesophageal echo, operative technique, and follow-up [Abstract]. J Am Coll Cardiol. 1993. 21:342A.
14. Lau LS, Blanchard DG, Hye RJ. Diagnosis and management of patients with peripheral macroemboli from thoracic aortic pathology. Ann Vasc Surg. 1997. 11:348–353.

15. Hahn TL, Dalsing MC, Sawchuk AP, Cikrit DF, Lalka SG. Primary aortic mural thrombus: presentation and treatment. Ann Vasc Surg. 1999. 13:52–59.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download