Abstract
Background
Spontaneous echo contrast (SEC) has been considered as a predisposition to thromboembolism and cerebrovascular accident. However, there have been few reports on the prevalence and role of SEC in dilated cardiomyopathy (DCM). The aim of this study was to investigate the prognostic usefulness of SEC in predicting a stroke in patients with DCM.
Methods
Between October 2001 and January 2008, transthoracic echocardiography with tissue harmonic imaging (THI) was performed for recognition of SEC in patients with DCM. Patients were divided into 2 groups according to the presence of SEC. Clinical characteristic data, echocardiographic parameters were compared between two groups.
Results
In this retrospective study, 220 patients (136 men, age 62.8±15.4 years) with DCM (left ventricular ejection fraction 27.8±7.8%) were included. SEC in the left ventricle (LV) was observed in 24 patients (10.9%). Stroke occurred in 4 (16.7%) of patients with SEC and in 9 (4.6%) of patients without SEC. There were no differences in LA dimension (p=0.24) and LV end-diastolic dimension (p=0.88) between both groups. On univariate analysis, SEC and coronary heart disease at presentation had statistical significance of risk factors for stroke in these groups (p<0.05). On multivariate analysis, only SEC was an independent predictor for stroke (OR 4.393, 95% CI 1.116-17.290, p=0.03).
Spontaneous echo contrast (SEC) is a smokelike echo phenomenon with a swirling pattern of blood flow that can be observed in transesophageal echocardiography (TEE) or transthoracic echocardiography (TTE), most often within the left atrium or left ventricle.1-3) SEC is first described by Feigenbaum in 1975,4) which has been observed under conditions of low blood flow velocity, such as rheumatic mitral stenosis, mitral valve prosthesis, atrial fibrillation (AF), dilated left atria and dyskinetic segments of the left ventricle (LV).5)6) SEC has been reported in 16% to 19% of patients selected to undergo TEE for standard indications. Dilated cardiomyopathy (DCM) probably is the end result of myocardial damage produced by various causes and shows various clinical manifestations.7)8) One of various clinical manifestations is SEC. However, there have been few reports on the prevalence of SEC in DCM.
SEC is significantly associated with a history of embolism and left atrial (LA) or LV thrombi detected by echocardiography, severity of mitral stenosis, reduced cardiac index, advanced age, atrial fibrillation, hypertension, heart failure, and large left atrial diameter. SEC is a cardiac factor most strongly associated with left atrial appendage thrombi and embolic events.1-3) But, there have been few reports on the role of SEC in DCM.
Currently, TTE is the standard procedure for detection of left ventricular (LV) thrombi9)10) with a sensitivity ranging from 92% to 95% and a specificity of 95% assuming a good image quality.9) In daily practice, however, poor image quality occurs in about 10% to 20% of patients,11) particularly at the apex, as a result of ultrasound artifacts and clutter. Tissue harmonic imaging (THI) reduces near-field and side-lobe artifacts,12)13) improving visualization of the blood/tissue interface and detection of the endocardial borders, especially in distorted LV.14)15)
Identification of thrombi or SEC by echocardiography in patients with DCM can be difficult in the presence of trabeculations, papillary muscles, echocardiographic artifacts, and poor near-field visualization.18) The importance of thrombi or SEC in patients with DCM is uncertain.20) Therefore, a reevaluation of the detection of SEC and its prognostic significance in DCM by THI through TTE is warranted.
The aims of our study were to investigate the relation between echocardiographic finding of SEC and the clinical outcomes such as stroke and death in patients with DCM and to clarify the prognostic usefulness of SEC in predicting stroke in patients with DCM.
In this retrospective study, from October 2001 to January 2008, 220 patients with DCM were studied by TTE with THI. These patients in functional class NYHA II-IV, diagnosed according to WHO criteria21) were recruited for the study. DCM was defined by LV dilation (LV end diastolic dimension ≥55 mm) and systolic dysfunction (LV ejection fraction ≤40%). All patients were divided into 2 groups according to the presence of SEC. Clinical characteristic data, echocardiographic parameters were compared between two groups. Data were collected including the presence or absence of atrial fibrillation, hypertension, diabetes, dyslipidemia [low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride], heart failure, ischemic heart disease, alcohol, and smoking history. We evaluated stroke in patients after detecting SEC by TTE with THI. Experienced neurologist performed neurological examination and diagnosed stroke which was based on their clinical history, neurologic sequelae and radiographic correlation. Stroke was defined acute onset of neurologic dysfunction of any severity consistent with focal brain ischemia and imaging/laboratory confirmation of an acute vascular ischemic pathology [Imaging/laboratory confirmation includes neuroimaging studies demonstrating recent, appropriately located ischemic lesion (DWI, CT), vascular imaging demonstrating an acute arterial occlusion or stenosis appropriate to the clinical syndrome (transcranial Doppler, MRA, CT angiography, conventional angiography) or perfusion technique demonstrating a perfusion deficit in an appropriately located vascular distribution (perfusion-weighted MRI, perfusion CT, single photon-emission CT, positron-emission tomography, xenon CT)].19) Patients were examined with CT and MRI for detection of cerebral infarction.
Our study was approved by the investigation and Ethics Committee of our institution.
Comprehensive 2-dimensional Doppler echocardiographic studies (PHILIPS Sonos 5500, 1 to 3 MHz; Philips Medical Systems and GE VIVID 7, 2.4 MHz; GE Medical Systems) were performed in all patients. M-mode echocardiographic measurements were made according to the American Society of echocardiography recommendation,22) and LV ejection fraction was calculated using the Quinones method. Five consecutive beats were averaged for all measurements. With standard transthoracic echocardiographic assessment of the 4 cardiac chambers and valves, the main cavities were closely inspected for evidence of thrombi or SEC. Harmonic mode denotes that the imaging system is programmed to transmit at one frequency and receive at twice that frequency, its second harmonic, using the same transducer. Spontaneous echocontrast was diagnosed in the presence of dynamic smoke-like echoes within the heart cavities with a characteristic swirling motion that was distinct from white noise artifact. Gain settings were adjusted as required to distinguish SEC from echoes due to excessive gain. SEC was graded as mild or severe according to the following: Near gain was increased progressively to just below the level at which static background noise appeared (threshold gain). Mild SEC was undetected at low gain and was seen in some parts of the left atrium or left ventricle at the threshold gain. Severe SEC occupied the entire atrium or ventricle and appeared very dense even at low gain. All echocardiograms were reviewed by 3 experienced observers with regard to presence or absence of SEC. When discrepancies occurred, echocardiograms were reviewed until a consensus was reached. But, in our study, all of SEC were diagnosed without thrombi in the LV and were not graded, just categorized as 'present' or 'absent' by THI in this study due to the limitation of retrospective study.
Differences in the prevalence of SEC in various subgroups were determined from 2×2 contingency tables using the chi-square or Fisher's exact tests. The relation of patient age, sex and the various stroke risk factors to the presence of SEC was assessed using multivariate logistic regression analysis. A multivariate logistic regression analysis with using SPSS 13.0 for Windows (SPSS inc., Chicago, IL) was used to determine the importance of the association of thromboembolic events with patient age, SEC and the other stroke risk factors. Values are expressed as mean±SD. Mean values were compared using the unpaired Student's t test. A p value<0.05 was considered significant.
Baseline characteristics of patients with and without SEC are shown in Table 1. This study included 220 consecutive patients (136 men and 84 women, average age 62.8±15.4 years) with DCM (mean ejection fraction 27.8±7.8%). SEC in the LV was observed in 24 patients (10.9%) (Fig. 1). The proportions of male (p=0.03), atrial fibrillation (p=0.05) and stroke (p=0.04) were significantly higher in patients with SEC than in patients without SEC. Age, hypertension, diabetes mellitus, total cholesterol, triglyceride, HDL-C, LDL-C, coronary heart disease, smoking and alcohol ingestion were not significantly different between both groups. The mortality rate was not different between patients with and without SEC (p=0.67).
Univariate analysis relating echocardiographic findings to the presence of SEC is shown in Table 2. There were no significant differences of LA dimension (p=0.24) and LV end-diastolic dimension (p=0.88) between patients with and without SEC. There were no significant differences of LV ejection fraction between two groups (p=0.75). There were no significant differences of other echocardiographic findings such as the presence of mitral regurgitation (p=0.80), tricuspid regurgitation (p=0.50) and tricuspid regurgitation pressure gradient (p=0.43) between two groups.
Of the risk factors to presence of SEC, gender and atrial fibrillation were statistically significant in univariate analysis (p<0.05) (Table 1). On multivariate logistic regression, there were no significant differences between the presence of SEC and the clinical risk factors of stroke such as diabetes mellitus (p=0.24), hypertension (p=0.48), dyslipidemia (p=0.07), coronary heart disease (p=0.18). Especially, there was no significant difference between atrial fibrillation and the presence of SEC (p=0.068) in this study.
Stroke occurred in 4 (16.7%) of patients with SEC and in 9 (4.6%) of patients without SEC. On univariate analysis, SEC and coronary heart disease at presentation had statistical significance of risk factors for stroke in these groups (p<0.05) (Table 3). When traditional risk factors of stroke were entered into multivariate logistic regression, patients with SEC by THI on TTE had a significantly higher occurrence rate of stroke [Odds Ratio (OR) 4.393, 95% Confidence Intervals (CI) 1.116-17.290, p=0.03] (Table 4). However, traditional risk factors of stroke such as hypertension, diabetes mellitus, dyslipidemia, AF, coronary heart disease, smoking, and alcohol ingestion were not statistically significant between patients with and without stroke.
Sixteen patients with SEC already had taken the antiplatelet or anticoagulant medications (e.g. aspirin: n=11, cilostazol: n=1, ticlopidine: n=1, heparin: n=3, warfarin: n=7). However, the incidence of previous antiplatelet or anticoagulation therapies was not different between patients with stroke and without stroke [75% (3/4) vs. 65% (13/20), p=0.69].
Our study constitutes the multivariate analysis that incorporates the information provided by TTE with THI in the last few years on the different factors associated with the presence of SEC in patients with DCM. This study is the first to demonstrate an association between the diagnosis of stroke in patients with moderate to severe LV dysfunction and the presence of SEC by TTE with THI. Previous studies have shown that patients with DCM are at high risk for stroke and embolic complications.23)24) However, there have been few reports on the prevalence and role of SEC in DCM. Therefore, this study may help in the risk stratification of cardiac embolism in patients with DCM.
Kozdag et al.25) reported impaired systolic function, restrictive filling pattern, presence of moderate to severe left atrial SEC, and complex atherosclerosis in the aorta are the factors contributing to the development of silent cerebral infarction. Gottdiener et al.23) reported higher rates of systemic embolization and insignificant trend toward increased mortality in patients with LV thrombi. Others have also suggested that patients with severe LV dysfunction tend to have higher rates of LV thrombi and systemic embolization.26) In a recent study of patients with severe LV dysfunction, the stroke rate was 14.9% in patients with and 9.5% in those without LV thrombi, but this was not statistically significant.20) There is no clear consensus regarding therapies (in particular, anticoagulation) in patients with DCM and LV thrombi.27)
The pathogenesis of SEC is not clearly established. However, it appears that multiple factors [e.g., aging, low blood flow velocity, high erythrocyte sedimentation (ESR), increased serum fibrinogen level, elevated hematocrit, structural abnormalities of cardiovascular system] potentially contribute to red blood cell and plasma protein interactions that lead to the development of SEC.28) Even though additional factors such as mitral regurgitation, hypercoagulability and elevated hematocrit may lead to development of SEC, LV systolic dysfunction may also predispose SEC with low flow rate rates and low shear rates. SEC is a cardiac factor most strongly associated with LV thrombi and embolic events. Therefore, the determination of the presence of SEC in DCM patients has important implications for prognostic and therapeutic usefulness.
Recent studies have demonstrated that THI without a contrast agent improves delineation of the left ventricular endocardial borders29-31) and the LA appendage16) by TTE. However, the efficacy of THI in the detection of SEC in DCM patients has not been fully evaluated. But Ha et al.32) demonstrated that THI significantly enhances the detection of LA SEC in patients with MS. One possible explanation for the enhanced visualization of LA SEC by THI is that a nonlinear emission of harmonics is produced by resonant aggregates of red blood cells, platelets, and plasma proteins similar to microbubbles.32) Harmonic imaging, which involves transmitting at the resonant frequency of the bubbles and constructing an image from the harmonic component of the returning ultrasound from the bubbles, eliminates the noise of the ultrasound reflections from static tissue and gives an increased signal-to-noise ratio.29) Although the met-hod for determining the presence of SEC by transthoracic THI has not yet been established, transthoracic THI has almost the same ability as TEE in the detection of severe LA SEC, which is known to be more strongly associated with LA thrombi and thromboembolism.32)33) And several studies showed the superiority of THI versus transthoracic fundamental imaging to detect left atrial, appendage and ventricle thrombi or SEC.16)17)
By improving image quality, THI may be used in the follow-up observational studies of SEC in a variety of clinical conditions. It also may be used in a serial observational study regarding the effects of anticoagulation or antiplatelet therapy on the appearance of SEC.
Our study is retrospective and possible confounding factors cannot be controlled. In this study, we did not take methods such as blinding of the echocardiographic reviewers to clinical data, so we could not minimize such retrospective bias. The number of patients with SEC (n=24) was small. And, TEE was not performed in these patients, so we could not compare the presence of SEC using TTE with that using TEE.
Figures and Tables
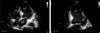
Fig. 1
Apical 4-chamber view with tissue harmonic imaging through transthoracic echocardiography in patient with dilated cardiomyopathy shows diffuse spontaneous echo contrast in left ventricle (LV). LA: left atrium.
References
1. Chimowitz MI, DeGeorgia MA, Poole RM, Hepner A, Armstrong WM. Left atrial spontaneous echo contrast is highly associated with previous stroke in patients with atrial fibrillation or mitral stenosis. Stroke. 1993. 24:1015–1019.

2. Fatkin D, Kelly RP, Feneley MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994. 23:961–969.

3. Zotz RJ, Müller M, Genth-Zotz S, Darius H. Spontaneous echo contrast caused by platelet and leukocyte aggregates? Stroke. 2001. 32:1127–1133.

4. Feigenbaum H. Coronary artery disease. Echocardiography. 1975. 2nd ed. Philadelphia: Lea and Febiger;341–380.
5. Castello R, Pearson AC, Labovitz AJ. Prevalence and clinical implications of atrial spontaneous contrast in patients undergoing transesophageal echocardiography. Am J Cardiol. 1990. 65:1149–1153.

6. Fatkin D, Herbert E, Feneley MP. Hematologic correlates of spontaneous echo contrast in patients with atrial fibrillation and implications for thromboembolic risk. Am J Cardiol. 1994. 73:672–676.

7. Song WH, Lim HE, Shin SH, Lee EM, Hwang KS, Ahn JC, Lim DS, Park CG, Shim WJ, Oh DJ, Ro YM. Changes of mitral inflow according to position in patients with dilated cardiomyopathy. J Korean Soc Echocardiogr. 1998. 6:5–10.

9. Visser CA, Kan G, David GK, Lie KI, Durer D. Two dimensional echocardiography in the diagnosis of left ventricular thrombus: a prospective study of 67 patients with anatomic validation. Chest. 1983. 83:228–232.

10. Arvan S. Mural thrombi in coronary artery disease: recent advances in pathogenesis, diagnosis, and approaches to treatment. Arch Intern Med. 1984. 144:113–116.

11. Shaw LJ. Impact of contrast echocardiography on diagnostic algorithms: pharmacoeconomic implications. Clin Cardiol. 1997. 20:I39–I48.

12. Thomas JD, Rubin DN. Tissue harmonic imaging: why does it work? J Am Soc Echocardiogr. 1998. 11:803–808.

13. Rubin DN, Yazbek N, Garcia MJ, Stewart WJ, Thomas JD. Qualitative and quantitative effects of harmonic echocardiographic imaging on endocardial edge definition and side-lobe artifacts. J Am Soc Echocardiogr. 2000. 13:1012–1018.

14. Mele D, Campana M, Sclavo M, Seveso G, Aschieri D, Nesta F, D'Aiello I, Ferrari R, Levine RA. Impact of tissue harmonic imaging in patients with distorted left ventricles: improvement in accuracy and reproducibility of visual, manual and automated echocardiographic assessment of left ventricular ejection fraction. Eur J Echocardiogr. 2003. 4:59–67.

15. Mele D, Teoli R, Cittanti C, Pasanisi G, Guardigli G, Levine RA, Ferrari R. Assessment of left ventricular volume and function by integration of simplified 3D echocardiography, tissue harmonic imaging and automated extraction of endocardial borders. Int J Cardiovasc Imaging. 2004. 20:191–202.

16. Ono M, Asanuma T, Tanabe K, Yoshitomi H, Shimizu H, Ohta Y, Shimada T. Improved visualization of the left atrial appendage by transthoracic 2-dimensional tissue harmonic compared with fundamental echocardiographic imaging. J Am Soc Echocardiogr. 1998. 11:1044–1049.

17. Mele D, Soukhomovskaia O, Pacchioni E, Merli E, Avigni N, Federici L, Levine R, Ferrari R. Improved detection of left ventricular thrombi and spontaneous echocontrast by tissue harmonic imaging in patients with myocardial infarction. J Am Soc Echocardiogr. 2006. 19:1373–1381.

18. Meltzer RS, Visser CA, Fuster V. Intracardiac thrombi and systemic immobilization. Ann Intern Med. 1986. 104:689–698.
19. Kidwell CS, Warach S. Acute ischemic cerebrovascular syndrome: diagnostic criteria. Stroke. 2003. 34:2995–2998.
20. Sharma ND, McCullough PA, Philbin EF, Weaver WD. Left ventricular thrombus and subsequent thromboembolism in patients with severe systolic dysfunction. Chest. 2000. 117:314–320.

21. Richardson P, McKenna W, Bristow M, Maisch B, Mauter B, O'connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996. 93:841–842.

22. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978. 58:1072–1083.

23. Gottdiener JS, Gay JA, Van Voorhees L, DiBianco R, Fletcher RD. Frequency and embolic potential of left ventricular thrombus in dilated cardiomyopathy: assessment by 2-dimensional echocardiography. Am J Cardiol. 1983. 52:1281–1285.

24. Fuster V, Halperin JL. Left ventricular thrombi and cerebral embolism. N Engl J Med. 1989. 320:392–394.

25. Kozdag G, Ciftci E, Vural A, Selekler M, Sahin T, Ural D, Kahraman G, Agacdiken A, Demirci A, Komsuoglu S, Komsuoglu B, Fici F. Silent cerebral infarction in patients with dilated cardio-myopathy: echocardiographic correlates. Int J Cardiol. 2006. 107:376–381.

26. Falk RH, Foster E, Coats MH. Ventricular thrombi and thromboembolism in dilated cardiomyopathy: a prospective follow-up study. Am Heart J. 1992. 123:136–142.

27. Hunt SA, Baker DW, Chin HM, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russell RO, Smith SC Jr. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary a report of the American college of cardiology/American heart association task force on practice guidelines (committee to revise the 1995 guidelines for the evaluation and management of heart failure): developed in collaboration with the international society for heart and lung transplantation: endorsed by the heart failure society of America. Circulation. 2001. 104:2996–3007.

28. Ansari A, Maron BJ. Spontaneous echo contrast and thromboembolism. Hosp Pract (Minneap). 1997. 32:109–111. 115–116.

29. Belohlavek M, Tanabe K, Mulvagh SL, Foley DA, Greenleaf JF, Seward JB. Image enhancement by noncontrast harmonic echocardiography: part II. Quantitative assessment with use of contrast-to-speckle. Mayo Clin Proc. 1998. 73:1066–1070.

30. Main ML, Asher CR, Rubin DN, Odabashian JA, Cardon LA, Thomas JD, Klein AL. Comparison of tissue harmonic imaging with contrast (sonicated albumin) echocardiography and Doppler myocardial imaging for enhancing endocardial border resolution. Am J Cardiol. 1999. 83:218–222.

31. Hwang JH, Yang DH, Shin SC, Cho S, Kim TI, Park HS, Cho YK, Chae SC, Jun JE, Park WH. Value of tissue harmonic imaging for the left ventricular wall imaging. J Kor Soc Echocardiogr. 2000. 8:198–205.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download