Abstract
A 69-year-old man was admitted to undergo percutaneous coronary intervention (PCI) for chronic total occlusion of right coronary artery. He had diabetes mellitus, stable angina pectoris. Diagnostic coronary angiography demonstrated proximal total occlusion of right coronary artery. PCI was failed due to failure of balloon passage. Echocardiography was performed after PCI and thickened epicardial tissue at right atrioventricular groove was noted. It was highly echogenic and localized along the course of mid right coronary artery. In following echocardiogram after 12 days, the size of echogenic mass was decreased from 3.4 cm×2.6 cm to 1.7 cm×0.7 cm and we could conclude it was right coronary artery hematoma associated with PCI.
Coronary artery perforation is a rare complication during or after percutaneous coronary intervention (PCI) and presents variably from hemodynamically stable concealed hemorrhage to potentially life-threatening cardiac tamponade. Here, we report a patient with coronary artery hematoma associated with coronary artery perforation after failed PCI for chronic total occlusion of right coronary artery, detected on transthoracic echocardiography (TTE).
A 69-year-old man was admitted to our institute with recently aggravated chest pain. He had history of diabetes mellitus. He felt exertional chest pain 6 years ago and was evaluated with coronary angiography which revealed chronic total occlusion of right coronary artery. Medical treatment was initiated and his symptom disappeared thereafter. But, recently he began to have recurrent episodes of exertional chest pain. Exercise stress test was performed and ST segment depression was noted in leads II, III, aVF and V3-6 with typical chest pain. In coronary angiogram, there was 90% stenosis of proximal left anterior descending artery and PCI with stenting was performed.
On physical examination, there were no significant findings. Laboratory studies showed no significant abnormalities. The electrocardiogram revealed signs of left atrial enlargement and first degree atrioventricular block with sinus bradycardia. The thoracic roentgenogram showed borderline cardiomegaly. In coronary angiogram, proximal part of the right coronary artery was totally occluded and had collateral flow from left anterior descending artery and left circumflex branch. To perform PCI to stenotic lesion of proximal right coronary artery, we inserted guidewire to proximal right coronary artery but guidewire did not pass through proximal right coronary artery. So we inserted the guidewire to distal right coronary artery using retrograde approach via septal branch of left anterior descending artery. Balloon dilatation underwent using 2.0 mm×15 mm balloon but PCI was failed because the balloon could not pass through the lesion in the mid right coronary artery. Final coronary angiogram showed no extravasation of contrast (Fig. 1).
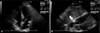
TTE was performed after PCI. The size, shape and function of left ventricle were normal. However, at the right atrioventricular groove, 3.4 cm×2.6 cm-sized prominent epicardial tissue with high echogenecity was noted. The echogenecity of the lesion was higher than that of the pericardial fat, so we interpreted this lesion as a localized hematoma (Fig. 2). As the patient showed stable vital sign and there was no sign of pericardial effusion in TTE, we managed patient conservatively with close observation. In following TTE after 12 days, the size of echogenic mass was decreased to 1.7 cm×0.7 cm and we could conclude it as a hematoma along with right coronary artery associated with PCI (Fig. 3). The patient was discharged in stable condition and managed medically on outpatient clinic.
Acute complications of PCI include acute coronary occlusion due to thrombus or coronary artery dissection, no-reflow, and coronary artery perforation. Although coronary artery perforation is not common, it results in potentially deadly complication such as cardiac tamponade and acute myocardial infarction.1)2)
The reported incidence of coronary artery perforations after PCI ranged from 0.1% to 0.6%,3-7) and increased recently as the use of debulking devices, such as, directional coronary atherectomy or rotational atherectomy became more common.8) The risk of coronary artery perforation is known to increase in the case of increased balloon to artery diameter ratio more than 1.1, PCI to heavily calcified or resistant stenosis, using high pressure inflation, using ablative devices such as rotablator, and using stiff hydrophilic wires in tortuous anatomy and chronic total occlusions.9)
Coronary artery perforation can be classified according to the findings of coronary angiography. Type I perforation is defined as the development of an extraluminal crater without extravasation, type II as a pericardial or myocardial blush without contrast jet extravasation, type III as extravasation through frank (>1 mm) perforation, and cavity spilling as perforation into an anatomic cavity chamber.10) Perforation grade is important because it is closely related to clinical outcome. Aamir et al. reported that cardiac tamponade, emergency coronary artery bypass graft (CABG), and in-hospital death were increased if the perforation grade was higher.9)
The management of coronary artery perforation after PCI is not well established. The suggested management strategy is entirely based on the experience of experts with large clinical experiences. According to the guideline, perforation grade and hemodynamic status of the patient determine the treatment plan. With grade I perforations, medical management with close observation is recommended if vessel diameter is smaller than 2 mm. If diameter is larger than 2 mm, consideration of stent deployment is recommended.11) If perforation grade is II or III, heparin should be immediately discontinued and systemic administration of protamine should also be attempted unless the situation prohibits it.1)12) Blood flow of the perforated branch should be blocked by using a balloon. In the meantime, TTE should be performed to check if cardiac tamponade develops. If TTE shows the presence of a pericardial fluid and sufficient pericardial space for safe centesis, pericardiocentesis must be carried out.13) If above-mentioned methods fail, emergency CABG should be considered.1)2) In selective cases, delivery of the covered stent might be attempted as an optional treatment. However, the restenosis rate of this device is known to be high. When the risk associated with the surgical procedure is considered to be higher than that with the restenosis or reocclusion of the covered stent, or when an emergent operation cannot be performed, this method is considered to be valuable.13)
In this case, we diagnosed coronary artery hematoma in a patient with hyperechoic mass at right atrioventricular groove on TTE after failed PCI for chronic total occlusion of right coronary artery. Although there were no myocardial blush or extravasation in coronary angiogram, echocardiographic finding of hyperechoic mass suggestive of right coronary hematoma meant existence of extravasation due to coronary perforation. As there was no pericardial fluid on TTE and vital signs of the patient were stable, we managed patient conservatively. So we report a case of successful diagnosis and observation of coronary artery perforation using TTE.
Figures and Tables
Fig. 1
Coronary angiography. A: Guidewire was inserted to distal right coronary artery via septal branch of left anterior descending artery. However balloon catheter (white arrow) could not pass through mid right coronary artery. Left anterior oblique cranial view. B: After percutaneous coronary intervention, final coronary angiogram was acquired. There was no extravasation of contrast. Left anterior oblique cranial view.
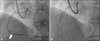
Fig. 2
Transthoracic echocardiography on the day of percutaneous coronary intervention. A: Homogeneous highly echogenic mass (white arrow) at the right atrioventricular groove was noted on apical four chamber view. B: The mass at the atrioventricular groove (white arrow) was about 3.4 cm×2.6 cm on subcostal view.
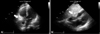
Fig. 3
Transthoracic echocardiography performed on 12 days after percutaneous coronary intervention. A: There was no visible mass at the right atrioventricular groove on apical four chamber view. B: The size of the mass (white arrow) was decreased from 3.4 cm×2.6 cm to 1.7 cm×0.7 cm on subcostal view.
References
1. Fejka M, Dixon SR, Safian RD, O'Neill WW, Grines CL, Finta B, Marcovitz PA, Kahn JK. Diagnosis, management, and clinical outcome of cardiac tamponade complicating percutaneous coronary intervention. Am J Cardiol. 2002. 90:1183–1186.

2. Von Sohsten R, Kopistansy C, Cohen M, Kussmaul WG 3rd. Cardiac tamponade in the "new device era": evaluation of 6999 consecutive percutaneous coronary interventions. Am Heart J. 2000. 140:279–283.

3. Kimbiris D, Iskandrian AS, Goel I, Bemis CE, Gehl L, Owens J, Segal BL. Transluminal coronary angioplasty complicated by coronary artery perforation. Cathet Cardiovasc Diagn. 1982. 8:481–487.

4. Grollier G, Bories H, Commeau P, Foucault JP, Potier JC. Coronary artery perforation during coronary angioplasty. Clin Cardiol. 1986. 9:27–29.

5. Topaz O, Cowley MJ, Vetrovec GW. Coronary perforation during angioplasty: angiographic detection and demonstration of complete healing. Cathet Cardiovasc Diagn. 1992. 27:284–288.

6. Teirstein PS, Hartzler GO. Nonoperative management of aortocoronary saphenous vein graft rupture during percutaneous transluminal coronary angioplasty. Am J Cardiol. 1987. 60:377–378.

7. Nassar H, Hasin Y, Gotsman MS. Cardiac tamponade following coronary arterial rupture during coronary angioplasty. Cathet Cardiovasc Diagn. 1991. 23:177–179.

8. Ellis SG, Ajluni S, Arnold AZ, Popma JJ, Bittl JA, Eigler NL, Cowley MJ, Raymond RE, Safian RD, Whitlow PL. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994. 90:2725–2730.

9. Javaid A, Buch AN, Satler LF, Kent KM, Suddath WO, Lindsay J Jr, Pichard AD, Waksman R. Management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2006. 98:911–914.

10. Bittl JA, Ryan TJ Jr, Keaney JF Jr, Tcheng JE, Ellis SG, Isner JM, Sanborn TA. Coronary artery perforation during excimer laser coronary angioplasty. The percutaneous excimer laser coronary angioplasty registry. J Am Coll Cardiol. 1993. 21:1158–1165.
11. Klein LW. Coronary artery perforation during interventional procedures. Catheter Cardiovasc Interv. 2006. 68:713–717.

12. Del Campo C, Zelman R. Successful non-operative management of right coronary artery perforation during percutaneous coronary intervention in a patient receiving abciximab and aspirin. J Invasive Cardiol. 2000. 12:41–43.
13. Shirakabe A, Takano H, Nakamura S, Kikuchi A, Sasaki A, Yamamoto E, Kawashima S, Takagi G, Fujita N, Aoki S, Asai K, Yoshikawa M, Kato K, Yamamoto T, Takayama M, Takano T. Coronary perforation during percutaneous coronary intervention. Int Heart J. 2007. 48:1–9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download