Abstract
Infective endarteritis in the pulmonary artery is unusual. However, congenital heart disease such as patent ductus arteriosus (PDA) could be a predisposing factor of infective endarteritis. We report a patient with PDA complicated by infective endarteritis and large pulmonary artery vegetation. After three weeks of antibiotic treatment, the patient underwent surgical closure of the PDA and removal of the vegetation.
Infective endarteritis was a fatal complication of PDA and the most common cause of death. However, the death rate of infective endarteritis associated with PDA has decreased due to early treatment such as surgical closure of PDA and use of antibiotics. The more sensitive diagnostic methods is a color doppler echocardiography.
A 49-year-old woman, with no history of heart disease, was admitted to the hospital with a febrile sensation, fatigue, and weight loss (2 kg). Three months ago, she was diagnosed as having a stricture of the pulmonary artery and suspicious pneumonia. So, the patient was treated empirically with antibiotics at another hospital. However the symptoms did not improve. No further information was obtained from the past medical and family history.
On physical examination, the blood pressure was 130/80 mmHg, the heart rate was 68 beats/min and regular, and the body temperature was 38℃. On inspection, the conjunctivae were anemic. Cardiac auscultation revealed a continuous murmur (grade III) at the left parasternal border.
Laboratory investigations revealed a normocystic, normochromic anemia (Hemoglobin 9.66 g/dL). The white cell count, C-reactive protein and erythrocyte sedimentation rate were 5,960×109/L, 6.52 mg/dL and 21 mm/h, respectively. The renal and liver function tests were normal. The chest X-ray revealed cardiomegaly (C/T ratio=0.6). The chest CT showed a dilated pulmonary artery and thombus in the proximal side of left main pulmonary artery. An electrocardiogram demonstrated sinus rhythm and the minimal voltage criteria for left ventricular hypertrophy.
Transthoracic echocardiography (TTE) showed a 0.5 cm defect between the descending thoracic aorta and the main pulmonary artery and a 3.4×1.0 cm hypoechogenic movable vegetation attached to the wall of the main pulmonary artery (Fig. 1). The left atrium was enlarged (4.26 cm). However, the diameter of left ventricle was normal. TTE showed a diastolic filling pattern of impaired relaxation (E/E'=12.3).
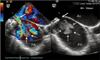
Transesophageal echocardiography (TEE) showed a 0.9 cm patent ductus arteriosus (PDA) and a left to right shunt flow through the PDA. A 3.4×2.1 cm multilobulated hypoechogenic pedunculated movable vegetation was found in the main pulmonary artery near the defect between the pulmonary artery and the aorta (Fig. 2).
The patient was treated with intravenous ceftriaxone (2.0 g every 24 hours) and gentamycin (60 mg every 8 hours). The body temperature was normalized. The vegetation decreased in size on the follow up TTE performed on the fifteenth hospital day. After 21 days of hospitalization, the patient underwent surgical closure of the PDA and resection of the vegetation. The post-operative TTE showed no shunt flow or vegetation. Three months after the surgery, the patient had no complications and was clinically in good condition.
PDA is a vascular structure that connects the proximal descending thoracic aorta to the roof of the main pulmonary artery near the origin of the left branch pulmonary artery.1) The incidence of PDA has been reported to be 0.02%-0.04% in live term births.2)
The most common complications associated with PDA are left heart failure and infective endarteritis.2) The development of endarteritis can be a fatal complication, it is the most common cause of death in patients with PDA.3)4) The echocardiographic detection of a vegetation is usually in the pulmonary artery near the end of the ductus, as was present in our case. The incidence of infective endarteritis associated with PDA has decreased significantly due to routine surgical closure of PDA and the widespread use of antibiotics. The incidence of infective endarteritis has been reported to be 1% per year and has progressively declined.1)5)
Predisposing factors for the development of infective endarteritis include intravenous drug abuse, sepsis, concomitant congenital heart disease and indwelling catheters. Two possible factors contribute to the development of vegetations and infective endarteritis caused by bacterial seeding in patients with PDA. One potential factor is the turbulence of blood flow between the aorta and the pulmonary artery. Another factor might be endothelial injury caused by turbulent blood flow. Seeding pathogens adhere to the injured arteries and the vegetation proliferates in the infected arteries.4)5)
Infective endarteritis can be diagnosed by clinical features, bacteremia confirmed by blood culture and echocardiography. Staphylococcal and Streptococcus viridans are the common microorganisms found in patients with PDA. However, culture negative infective endarteritis can occur in about 10% of all cases.6-8) In our case, bacteremia was not confirmed on the blood culture because the patient was treated with antibiotics at another hospital. Echocardiography has a high sensitivity and a high negative predictive value for the diagnosis of infective endarteritis. In adult patients, PDA with vegetations in the pulmonary artery is not easily visualized by TTE. TEE appears to be more sensitive and a superior diagnostic tool for the detection of PDA and vegetations.9)10)
In patients with infective endarteritis, bactericidal antibiotics are usually administered intravenously for two-six weeks. The PDA closure is clearly indicated for any child or adult who is symptomatic from significant left-to-right shunting through the PDA.1) But surgical or percutaneous closure of the PDA is not indicated in those patients with severe, irreversible pulmonary hypertension.2) However, patients with PDA and infective endarteritis should have a surgery. Because the infective endarteritis associated with congenital heart disease can cause severe complications such as cardiac failure, organ failure due to septic emboli, fatal arrhythmias and neurological problems such as a stroke or mycotic aneurysm.2)7)10)
This report illustrates the diagnosis and management of PDA with a vegetation and pulmonary artery endarteritis. After treatment with antibiotics and surgical closure of the PDA the patient is doing well.
Figures and Tables
Fig. 1
Suprasternal notch view of the transthoracic echocardiography shows a defect between the descending thoracic aorta and the main pulmonary artery (solid arrow) and a hypoechogenic multilobulated vegetation on the wall of the main pulmonary artery (dotted arrow). Ao: aorta, PA: pulmonary artery.
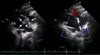
Fig. 2
Transesophageal echocardiography shows a turbulent blood flow from the aortic arch to the main pulmonary artery via patent ductus arteriosus (solid arrow) and hypoechogenic multilobulated vegetations attached to wall of the main pulmonary artery (dotted arrow). Ao: aorta, PA: pulmonary artery.
References
2. Gonzalez IC, Herrero FM, Sanchez JL. Infective endarteritis in patent ductus arteriosus and septic pulmonary embolism. Rev Esp Cardiol. 2006. 59:397–401.

3. Kouris NT, Sifaki MD, Kontogianni DD, Zaharos I, Kalkandi EM, Grassos HE, Babalis DK. Patent ductus arteriosus endarteritis in a 40-year old woman, diagnosed with transesophageal echocardiography. A case report and a brief review of the literature. Cardiovascular Ultrasound. 2003. 1:2.

4. Ozkokelli M, Ates M, Uslu N, Akcar M. Pulmonary and aortic valve endocarditis in an adult patient with silent patent ductus arteriosus. Jpn Heart J. 2004. 45:1057–1061.

5. Kim SH, Woo HY, Ha JH, Kim CW, Choi YS, Rhee DH, Kim JH, Park CS, Oh YS, Youn HJ, Chung WS, Hong SJ. A case of patent ductus arteriosus associated with pulmonary valve endocarditis. JCU. 2006. 14:33–35.

6. Kim SH, Huh J, Kang IS, Lee HJ, Yang JH, Jun TG, Park PW. Infective endocarditis in adolescents and adults with congenital heart disease. Korean Circulation J. 2006. 36:318–323.

8. Noh CI. Infective endocarditis in congenital heart disease patients: it's time to pay more attention. Korean Circulation J. 2006. 36:252–254.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download