Introduction
Spontaneous native aortic valve thrombosis is a rare condition. Almost all published cases have been associated with heart valve disease, heart valve replacement, a hypercoagulative state or the presence of an autoimmune disease.1) The present case is unique in that a thrombus developed on a native aortic valve with no predisposing cause, which resulted in a potentially fatal embolism to a coronary artery.
Case
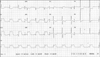
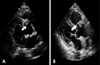
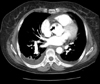
A 61-year-old woman visited our hospital complaining of chest pain for two hours duration. The patient had a blood pressure of 80/60 mmHg, a pulse rate of 66 beats/min and a respiratory rate of 22 breaths/min. A chest X-ray revealed a normal-sized heart and alveolar edema. Initial electrocardiography (ECG) showed ST-segment elevation on leads II, III and aVF (Fig. 1). Laboratory findings included an increased white blood cell count of 15,880/㎕ and a normal hematocrit and platelet counts. The levels of cardiac enzymes were elevated with a troponin-T level of 0.98 ng/㎕ (normal: 0.00-0.10 ng/㎕) and creatine kinase-MB (CK-MB) level of 10 ng/㎕ (normal: 0.1-4.94 ng/㎕). The blood tests showed no evidence of a hypercoagulable state, including protein C and S deficiency or antithrombin deficiency. Transthoracic echocardiographic findings showed akinesis of the left ventricular inferior wall segment without ventricular wall thinning with a left ventricular ejection fraction of 64%. Transthoracic echocardiography also showed the presence of an approximately 1.5×1.2 cm sized soft echocardiographic mass attached to the right coronary cusp of the aortic valve, near the right coronary artery orifice (Fig. 2). The aortic valve was a normal tricuspid without calcification. The chest and abdominal computed tomographic (CT) findings showed a 1.6 cm sized mass above the aortic valve with no evidence of a pulmonary embolism or deep vein thrombosis (Fig. 3).
We could not conduct a primary coronary intervention because of the risk of a catheter-induced rupture of the thrombus and a subsequent systemic embolism. We recommended open-heart surgery but the patient refused. Instead, the patient was started with thrombolytic therapy with the use of Tenecteplase. After thrombolysis and hydration, chest pain was relieved and the blood pressure was normalized.
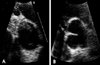
A subsequent echocardiogram performed after two days revealed that the thrombus had decreased in size and changed in shape (Fig. 4A).
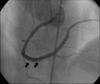
A final echocardiogram showed complete resolution of the thrombus of the aortic valve (Fig. 4B). A coronary angiogram revealed a small thrombus in the mid and distal right coronary artery (RCA) but there was no significant stenosis (Fig. 5). The left anterior descending artery (LAD) and the left circumflex artery (LCX) were normal. The patient was discharged in good condition and was managed with antiplatelet agents and warfarin.
Discussion
A spontaneous native aortic valvular thrombosis is a very rare condition. The first report of a native aortic valve thrombosis concerned a neonate following heart catheterization for coarctation.2) Thrombosis had resulted from trauma to the endothelium of the valve.
Native aortic valvular thrombosis usually follows local trauma such as cardiac surgery or catheterization,2) or it occurs as a complication of infection such as bacterial endocarditis3) or occurs in the presence of any hypercoagulable state such as protein S deficiency4)5) or antiphospholipid antibody syndrome. Circulating antibodies may have a particular affinity to the endothelium of the valve favoring autoimmune complex formation.6)
Valve dystrophy such as aortic stenosis may induce endothelial lesions that may trigger the mechanism of thrombosis when associated with abnormal blood flow.7-9) Local flow turbulence promotes repeated cycles of thrombus deposition, organization and re-endothelization with progressive thickening. The organization resulting from such thrombi may contribute to the continuous process of stenosis and deformation of the valve.9) We did not find any factor favoring the occurrence of the valve thrombosis in this patient. The valve was tricuspid, not dystrophic, had no functional abnormality, and the biochemical analyses for circulating antibodies, tumoral markers and coagulation disorders were negative.
Native valvular thrombus is difficult to differentiate from a tumor, and in particular, a papillary fibroelastoma. Both a native valvular thrombus and a papillary fibroelastoma can cause systemic embolic events. The presence of this valvular disease, regardless of size and shape, is an indication for prompt surgical resection, both to pathologically confirm the condition and to avoid the potential for life-threatening complications from a left-sided mass.8)10) In this case, we were unable to perform emergency surgery as the patient declined consent while primary coronary intervention for acute myocardial infarction was not indicated, given the possibility of the occurrence of a catheter induced systemic embolism. Serial echocardiographic findings revealed that the mass size was reduced over time and the mass in the aortic valve was confirmed as a thrombus.
In conclusion, this patient that presented with no risk factors and no previous history of thrombosis or valve dystrophy was diagnosed with an idiopathic native aortic valve thrombosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download