Abstract
Background
Previously, the authors revealed that epicardial fat thickness was significantly correlated with the severity of coronary artery disease in patients with known coronary artery disease. We evaluated whether echocardiographic epicardial fat thickness was associated with short term prognosis in patients with acute coronary syndrome.
Methods
Two hundred and sixty five consecutive acute coronary syndrome (ACS) patients who underwent successful coronary stenting were studied. Epicardial fat thickness on the free wall of right ventricle was measured at end-diastole from the parasternal long-axis views of three cardiac cycles. 30 days follow-up was obtained in all patients and clinical outcomes were compared with epicardial fat thickness.
Results
Mean value of epicardial fat thickness was 5.36 mm (range 0.44 to 16.55 mm). Major adverse cardiac events (MACE) were developed in 19 patients (7.2%) during 30 days; 2 cases of cardiac death, 11 of non fatal Q wave myocardial infarction (QMI), 4 of revascularization and 2 of ischemic stroke. Incidence of occlusion by thrombi (4.5% vs. 21.2%, p=0.016), Gensini's score (44.52±31.06 vs. 61.00±30.68, p=0.027) and epicardial fat thickness (5.19±2.13 vs. 7.51±3.87 mm, p=0.018) were significantly higher in patients with MACE than those without MACE. Significant correlations were demonstrated between epicardial fat thickness and age (r=0.193, p=0.002), fibrinogen (r=0.145, p=0.022) and LDL-cholesterol (r=0.136, p=0.027). Multivariate analysis showed that epicardial fat thickness (OR 1.479, 95% CI 1.183-1.848, p=0.001) was an independent predictor of 30 days MACE.
Metabolic syndrome is related to multiple cardiovascular risk factors.1-3) Visceral obesity seems to play a key role in the development of all features of metabolic syndrome.4-7) Hence, the detection of visceral adipose tissue, fat deposited around the internal organs, might be important for the risk stratification of metabolic syndrome. Several methods are applied as surrogates for estimation of visceral adipose tissue.
Epicardial adipose tissue is a visceral fat deposited around the heart, particularly around subepicardial coronary vessels. It may act as an endocrine organ given the production of a comparable pattern of adipocytokines8) and has been implicated in the development of coronary atherosclerosis.9) Recent reports have shown that epicardial adipose tissue expresses numerous genes for cytokines and proteins associated with atherosclerosis.8-10)
Previously, we proposed and validated that epicardial fat thickness was significantly correlated with the severity of coronary artery diseases in patients with known coronary artery disease.11) We hypothesized that the short term prognosis after acute coronary syndrome (ACS) was related with epicardial fat thickeness. To test this hypothesis, we compared epicardial fat thickness with short term clinical outcome between patients who underwent successful coronary stenting.
We included 265 consecutive patients who had ACS and received coronary stenting and whose epicardial fat thickness was measured by echocardiography on the next day after coronary angiography. The clinical diagnoses of patients on admission were unstable angina pectoris (UAP) in 199 (75.1%) and non ST elevation myocardial infarction (NSTEMI) in 66 (24.9%) patients. We excluded cases with severe degree of valvular heart disease and arrhythmia.
On admission, blood sampling was performed to measure routine chemistry including serum creatinine, total cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL cholesterol), low-density lipoprotein-cholesterol (LDL cholesterol), lipoprotein (a), fibrinogen and high-sensitivity C-reactive protein in overnight fasting state.
In a fasting state, coronary angiography was performed using the Judkins' method, following the puncture of the femoral artery or via a radial artery approach. The severity of coronary atherosclerotic lesions was evaluated from at least 3 projections in all the patients. Significant stenosis was defined as a diameter stenosis of 50% or greater.
The angiographic characteristics of the coronary atherosclerotic lesions were defined using Gensini's score.12) In this scoring system, a greater reduction of the luminal diameter was assigned a high score and a proximal lesion in the left anterior descending or the left circumflex artery was assigned a higher score than a distal lesion.
Percutaneous coronary intervention (PCI) was performed according to current clinical practice at the physician's discretion. PCI was performed immediately after diagnostic angiography. On diagnostic coronary angiography, the patency of the treated artery was evaluated by the Thrombolysis In Myocardial Infarction (TIMI) score. Angiographic success of PCI was defined as TIMI III flow with residual stenosis below 20%.
In all patients, aspirin (300 mg/day) and clopidogrel (300 mg/day) were loaded before procedure. An intravenous bolus of 5,000 U of unfractionated heparin was given, and then additional heparin boluses were given to maintain activated clotting time >300s during the procedure. Platelet glycoprotein IIb/IIIa inhibitors (GPI) were administered according to the operator's preference.
The occurrence of angiographic complications during PCI was recorded. These included failed PCI such as wire or balloon passage failure, side branch occlusion, slow or no reflow, major dissection, and distal embolization.
Each patient underwent transthoracic echocardiography the next day after coronary angiography. Echocardiograms were performed with a VIVID 7 (GE, USA) instrument according to standard techniques, with subjects in the left lateral decubitus position. Echocardiographic images were recorded onto a computerized database and videotape. The offline measurement of epicardial fat thickness was performed by 2 cardiologists who were unaware of the clinical and angiographic data.
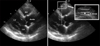
We measured epicardial fat thickness on the free wall of right ventricle from the parasternal long-axis views. Epicardial fat was identified as an echo-free space in the pericardial layers on the 2-dimensional echocardiography, and its thickness was measured perpendicularly on the free wall of the right ventricle at end-diastole for 3 cardiac cycles.13)14) In order to standardize the measuring point between different observers, we used the aortic annulus as anatomical reference. The measurement was performed at a point on the free wall of the right ventricle along the midline of the ultrasound beam, perpendicular to the aortic annulus (Fig. 1). The average value from 3 cardiac cycles for each echocardiographic view was used for the statistical analysis. The intra-observer and inter-observer correlation coefficient was 0.98 and 0.94, indicating good reproducibility and reliability.
The end point of this study was the occurrence of major adverse cardiac events (MACE) during in-hospital period and 30 days. MACE included cardiac death, non fatal myocardial infarction (MI) including postprocedural MI, revascularization, and ischemic stroke. Postprocedural MI defined as a postprocedural increase of biochemical markers of myocardial necrosis including creatinine kinase-MB (CK-MB) and troponin T (TnT) over 2 times higher than the normal upper limit in patients with normal baseline level. In patients with elevated baseline levels of cardiac enzyme, MI was defined as a subsequent increase by over 2 times in biomarkers from the baseline value and an additional increase in the second sample.15)16)
Continuous variables are expressed as mean±standard deviation. Discrete variables are presented as frequencies and percentages of those with data available. Comparisons between the groups were analyzed using independent t-test and χ2 test, which were conducted using SPSS 15.0 for Window (SPSS inc., Chicago, IL). A multivariable logistic regression model was constructed for the prediction of cumulative 30 day MACE. The following variables, selected according to the literature data17)18) and significant univariate analysis, were inserted into the logistic regression analysis; existent MI on admission, age, diabetes, smoking, serum creatinine, LDL-cholesterol, left ventricular ejection fraction, epicardial fat thickness, multivessel disease and Gensini's score. For continuous variables, the median value was used as a cut-off point to define the two subgroups in logistic regression analysis. Statistical significance was set at p<0.05.
The mean age of patients was 63.13±10.79 years and 167 (63.1%) of the subjects were male. The mean value of the epicardial fat thickness was 5.36 mm (range 0.44 to 16.55 mm). MACE were occurred in 19 patients (7.2%) during 30 days; 2 cases of cardiac death (0.8%), 11 of non fatal MI (4.2%) including 8 of postprocedural non Q-wave MI, 4 of revascularization (1.5%) and 2 of ischemic stroke (0.8%). We compared these with the patients without MACE.
The main clinical and laboratory findings of the 2 groups were summarized in Table 1. Age (62.70±10.77 vs. 68.57±9.66 years, p=0.022), present MI on admission (22.8% vs. 52.6%, p=0.004) and epicardial fat thickness (5.19±2.13 vs. 7.51±3.87 mm, p=0.018) were significantly higher in patients with MACE than in those without.
Table 2 shows the angiographic and procedural characteristics. Incidence of thrombotic occlusion was higher (4.5% vs. 21.1%, p=0.016) in the patients group with MACE. Gensini's score, which represents the severity of coronary artery lesion, was higher in patients with MACE than in those without.
As shown in Table 3, a significant correlation was revealed between epicardial fat thickness and age (r=0.193, p=0.002), fibrinogen (r=0.145, p=0.022) and LDL-cholesterol (r=0.136, p=0.027) was found. No significant correlation was found between epicardial fat thickness and the other variables (BMI, Glucose, hsCRP, Creatinine, etc).
The only independent factor affecting 30 days MACE was demonstrated through multivariate analysis was epicardial fat thickness (OR 1.479, 95% CI 1.183-1.848, p=0.001) (Table 4).
In this study, our results showed that epicardial adipose tissue detected by echocardiography was correlated with short term prognosis in patients with ACS.
Obesity is recognized as an important risk factor for the development of all features of metabolic syndrome and atherosclerotic cardiovascular disease. Regional distribution of body adipose tissue, rather than total body adiposisty, has increasingly gained attention as a marker for cardiovascular disease.4-7) Epicardial adipose tissue is a true visceral fat tissue, deposited around the heart on the free wall of the right ventricle, left ventricular apex and atrium. Previous reports indicated that epicardial adipose tissue was strongly correlated with abdominal fat deposits.14) In explaining of this finding, epicardial fat and intra-abdominal fat seem to be originally brown adipose tissue in infancy.
The biochemical properties of epicardial fat tissue suggested its possible role as a cardiovascular risk factor. Studies using epicardial fat obtained during coronary artery bypass surgery revealed significantly higher expression of interleukin-1, interleukin-6, and tumor necrosis factor mRNA in epicardial fat than in leg subcutaneous adipose tissue.8)10) Other studies revealed that epicardial and omental fat exhibited a comparable pathogenic inflammatory mRNA profile.9) Therefore, epicardial fat seemed to play a role as a local inflammatory burden and store in patients with coronary artery disease.
Hence, an estimation of epicardial adipose tissue is important, and several methods have been applied as a surrogate for the assessment of epicardial adipose tissue.13)14)19)20) Pericardial fat area, including epicardial adipose tissue, measured by thoracic CT was correlated significantly with the extent of coronary artery disease measured angiographically in both lean and overweight nondiabetic Japneses men.19) However, it is not clear to what extent epicardial adipose tissue area per se is correlated with coronary artery disease. In 2003, Iacobellis et al first reported the development of the echocardiographic measurement of epicardial fat.13)14) They showed that echocardiographic epicardial fat thickness had a good correlation with MRI abdominal fat and epicardial fat measurements, and anthropometric and metabolic parameters. However, there have been no reports of the correlation between epicardial adipose tissue and the presence and severity of coronary artery disease in a clinical setting.
Echocardiographic calculation of epicardial fat was easily reproducible and less expensive than MRI and computed tomography techniques. Also data by echocardiography was useful in the clinical management of patients with ACS. Our previous study demonstrated that epicardial fat thickness measured using echocardiography was significantly correlated with the severity of coronary artery disease.11) We hypothesized that the short term prognosis after acute coronary syndrome (ACS) was related with epicardial fat thickeness. In this study, we found a good correlation between epicardial adipose tissue and occurrence of MACE. Particularly, most of MACE was consisted with of MI including postprocedural MI, and this suggested that epicardial fat thickness was associated with MI. Although we could not make direct comparison of this association, some mechanisms may be evoked to explain this correlation. We could assume that, when epicardial fat augments, a long lasting exposure to inflammatory stimuli might be responsible for a gradual increase of inflammatory cytokines. An increased cytokines may contribute to a pro-atherogenic and prothrombogenic environment on the coronary arteries. Thus far, the direct contribution of fat surrounding the coronary arteries to the development of coronary atherosclerosis was associated with severity and prognosis of coronary artery diseases.
There are several limitations. Each treatment for ACS alters the atherogenic environment of the coronary arteries, but each individual patient receives different treatment. Further studies including long term follow-up are needed to generalize our result. Also further investigations with a larger population will be necessary to create threshold values of mild and severe epicardial adipose deposition.
In conclusion, epicardial fat thickness was significantly correlated with short term prognosis in patients with ACS. This suggests that echocardiographic epicardial fat thickness could be applied as predictive marker of prognosis in patients with ACS.
Figures and Tables
Fig. 1
Example of measurement of epicardial fat thickness. Epicardial fat was identified as an echo-free space in the pericardial layers on the two-dimensional echocardiography and its thickness was measured perpendicularly on the free wall of the right ventricle at end-diastole.
References
1. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988. 37:1595–1607.

2. DeFronzo RA, Ferrannini E. Insulin resistance. a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991. 14:173–194.

3. Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes. 1992. 41:715–722.

4. Doll S, Paccaud F, Bovet P, Burnier M, Wietlisbach V. Body mass index, abdominal adiposity and blood pressure: consistency of their association across developing and developed countries. Int J Obes Relat Metab Disord. 2002. 26:48–57.

5. Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord. 2001. 25:1730–1735.

6. Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA. 1998. 280:1843–1848.

7. Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord. 2001. 25:1047–1056.

8. Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003. 108:2460–2466.

9. Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006. 5:1.

10. Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, Gallo P, di Gioia CR. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005. 29:251–255.

11. Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK, Rhee SJ, Lee EM, Lee J, Yoo NJ, Kim NH, Park JC. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007. 71:536–539.

12. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease (Letter). Am J Cardiol. 1983. 51:606.

13. Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, Leonetti F. Epicardial fat from echocardiography: A new method for visceral adipose tissue prediction. Obes Res. 2003. 11:304–310.

14. Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003. 88:5163–5168.

15. Meier MA, Al-Badr WH, Cooper JV, Kline-Rogers EM, Smith DE, Eagle KA, Mehta RH. The new definition of myocardial infarction: diagnostic and prognostic implications in patients with acute coronary syndromes. Arch Intern Med. 2002. 162:1585–1589.
16. Roger VL, Killian JM, Weston SA, Jaffe AS, Kors J, Santrach PJ, Tunstall-Pedoe H, Jacobsen SJ. Redefinition of myocardial infarction: prospective evaluation in the community. Ciculation. 2006. 114:790–797.
17. Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, Akkerhuis KM, Harrington RA, Deckers JW, Armstrong PW, Lincoff AM, Califf RM, Topol EJ, Simoons ML. The PURSUIT Investigators. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. Circulation. 2000. 101:2557–2567.

18. Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA. GRACE Investigators. A validated prediction model for all forms of acute coronary syndrom: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004. 291:2727–2733.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download