Ischemic mitral regurgitation (MR) develops in approximately 20% of the patients following acute MI without papillary muscle (PM) rupture and 50% of those with congestive heart failure.1-5) Due to the large number of patients with acute MI, the incidence of ischemic MR is also high. Ischemic MR affects patients' prognosis, doubling mortality following myocardial infarction and heart failure.1-4) While only severe MR due to organic lesions has been shown to affect prognosis, even mild ischemic MR can significantly affect survival.3)4) In most patients, the grade of ischemic MR usually appears mild, so its importance may often be unrecognized by physicians. In addition, the treatment strategy is not established. Surgical as well as non-surgical interventions are effective, but considerable numbers of patients with ischemic MR can often present with persistent or recurrent MR even after therapeutic interventions. Therefore, ischemic MR presents a vexing clinical problem, which requires established therapeutic strategies based on mechanistic understanding.
Because MR is a mechanical phenomenon, MR must be associated with separation or weak contact between the anterior and posterior leaflets, which may reflect a morphological change of the leaflets. Since leaflets and chordae are largely avascular tissue and resistant to ischemia, alterations in other structures or hemodynamic changes may result in leaflet dysfunction/deformation and subsequent regurgitation. Potential changes that could affect leaflet function include alterations in the mitral annulus, the left ventricular (LV) free wall, the PM, and the pressure or flow around the leaflets. The first step to explore the mechanism of ischemic MR is the evaluation of the morphological characteristics of the leaflets.
Apical displacement of the leaflets relative to the annulus is an important characteristic of the configuration of the mitral leaflets. Apical displacement of the leaflets and the coaptation relative to the annulus in patients with ischemic MR was initially observed by Ogawa et al. and by Godley, Weyman, and colleagues (Fig. 1).6)7) In principle, the spatial position of the systolic leaflets and the coaptation can be determined by 2 superimposed and opposing forces acting on the leaflets. Increased LV pressure acts to push the leaflets toward the left atrium, while tethering force of the chordae pulls the leaflets to prevent leaflet prolapse into the left atrium. Therefore, systolic position of leaflets can be determined by the balance of these 2 forces (Fig. 2). Apical displacement of the leaflets and coaptation results from greater tethering force, which can be derived by reduced closing force and/or augmented tethering force. The tethering force is directly related to the position of the PMs, and outward displacement of the PMs can cause augmented tethering force. Because anterior annulus is spatially fixed at the aortic root, the distance between the anterior annulus and the PMs can be used as a measure to express the outward displacement of the PMs and tethering (Fig. 2).8) Measurement of the distance between the PM tip and the anterior annulus with apical views using 2-dimensional echocardiography can be practical in clinical patients (Fig. 3).9)10)
The reduction in the closing force with apical displacement of the leaflets has been reported as the main determinant of the ischemic MR.11)12) This was based on observations that patients with ischemic MR generally have LV dysfunction. An animal experiment with LV dysfunction has further demonstrated apical displacement of the leaflets.11) However, these observations have been performed in association with LV dysfunction and LV dilatation. Therefore, separation of LV dysfunction from dilatation is necessary to understand the relationship between these factors and the apical displacement of the leaflets.
He et al.13) developed an in vitro mitral valve model and observed that outward displacement of the PM caused MR with apical displacement of the leaflets in the absence of reduced closing force or LV pressure. Schwammenthal and colleagues created an in vivo model to separate LV dysfunction from dilatation, in which severe LV dysfunction was induced by beta-blockade with LV ejection fraction below 20% and the LV dilatation was controlled by pericardial restraint and decreasing preload. Investigators observed that severe LV dysfunction without significant LV dilatation failed to cause apical displacement of the leaflets and MR, while the combination of LV dysfunction and dilatation resulted in apical displacement of the leaflets with ischemic MR.14) The increase in the tethering distance between the PMs and the anterior annulus was proportional to the degree of the ischemic MR in this investigation. This study strongly suggests that augmented PM tethering secondary to LV dilatation is the main cause of ischemic MR.
This concept is consistent with the data by other investigators,15-17) including a report by Yiu and Sarano et al that characterized the relationship between PM tethering, leaflet tenting, and severity of ischemic MR in clinical patients.9) The importance of LV sphericity in the development of ischemic MR also supports the tethering concept as a mechanistic explanation for ischemic MR.18)19)
It should be noted that PM tethering is not necessarily equal or proportional to LV dilatation. This concept is consistent with the higher incidence of ischemic MR in patients with inferior myocardial infarction compared to those with anterior infarction and larger LV.20)21) Using an animal model, Gorman et al.22) have demonstrated that LV dilatation without prominent geometric changes in the mitral valve apparatus with anteroseptal MI does not cause ischemic MR, while posterior MI, with mitral valve annular and posteromedial PM geometric changes, causes MR in a sheep model (Fig. 4). Further, Kumanohoso et al.23) reported that inferior MI causes less global LV dilatation but greater displacement of the medial PM in clinical patients. Therefore, ischemic MR is proportional to the degree of deformity of the mitral valve complex, especially the outward displacement of the PMs, rather than to global LV dilatation.
If augmented tethering is the main cause of ischemic MR, the procedures to reverse the tethering would be expected to attenuate MR. Liel-Cohen and Guerrero et al.24) created an animal model of chronic ischemic MR with apical displacement of leaflets due to postero-lateral wall bulging underlying the medial PM. By plicating the bulging wall, the outward displacement of the medial PM was corrected, and the MR was also eliminated. Hung et al.25) have applied a localized patch containing an epicardial balloon over inferior infarcts with ischemic MR in a sheep model. Injection of saline into the balloon repositioned the underlying wall and PM and eliminated MR with leaflet tenting. Reverse remodeling procedures are now under active investigation in clinical patients.26-29)
Isolated severe LV dysfunction failed to cause significant ischemic MR,8)14) however, the role of reduced closing force still seems important as a supplemental factor leading to ischemic MR in the presence of augmented tethering force. In an in vivo model of ischemic MR caused by fixed outward displacement of the PM and constant annular dilatation, He et al. investigated the effects of LV pressure rise on the mitral regurgitation. Increase in systolic LV pressure in this model with fixed geometry of the mitral valve complex paradoxically reduced the apical displacement of the leaflets as well as the regurgitant fraction.13) Further, Schwammenthal et al.30) have found that ischemic MR dynamically changes in severity within a cardiac cycle, with the severity often maximal in early and late systole and minimal in mid-systole with maximal LV pressure (Fig. 5). Hung et al.31) also confirmed such dynamic MR even in patients with surgical ring annuloplasty, confirming the importance of closing force. Breithardt et al.32) have demonstrated that acute reduction in ischemic MR following cardiac re-synchronization treatment prior to chronic reverse LV remodeling is associated with increase in the leaflet closing force (LV + dP/dt). Fukuda et al.33) further demonstrated that CRT acutely reduces only the early systolic MR and has no acute effects on mid to late systolic MR. These data suggest that, although the closing force is not the primary determinant of ischemic MR, it may be significant especially in the presence of augmented leaflet tethering due to PM displacement.
The concept of PM dysfunction was based on clinical observations that ischemic MR occurred after inferior myocardial infarction and secondary dysfunction of the medial PM.34)35) Ischemic MR was expected to result from leaflet prolapse due to the reduced longitudinal contraction of the PMs secondary to ischemic dysfunction (Fig. 6). However, multiple experimental studies have reported that isolated PM dysfunction did not cause ischemic MR.36-40) In addition, clinical studies have also reported that leaflet prolapse in patients with ischemic MR is very rare.6)7)20) Therefore, the relationship between PM dysfunction and ischemic MR is not yet clear.
Recent reports suggest the central role of tethering in ischemic MR.6-10),13-19) Therefore, it is reasonable to view the effects of PM dysfunction on the mitral valve function from the standpoint of leaflet tethering. In this context, LV remodeling in the wall adjacent to the PM may result in augmented tethering and secondary MR (Fig. 7). This can be expressed as associated effects of PM dysfunction to augment tethering and ischemic MR. Alternatively, in the presence of LV remodeling in the adjacent wall, PM dysfunction per se may attenuate longitudinal PM shortening and tethering. This can be expressed as direct effects of PM dysfunction to attenuate tethering and MR.
This concept was initially explored by Messas et al. in an animal model of ischemic MR induced by basal posterior myocardial infarction with maintenance of PM perfusion to assure normal PM function. The investigators observed that addition of PM dysfunction with ischemia attenuated leaflet tethering and MR.41) This concept was later explored in clinical patients by Uemura et al.42) Since PM dysfunction may exert 2 opposing effects on mitral valve function, the relationship between PM dysfunction and MR is likely not linear. Associated LV adjacent wall remodeling to augment tethering may modulates direct effects of PM dysfunction to attenuate tethering and MR. In this case, PM dysfunction may be associated with less MR in selected patients with a similar degree and location of LV remodeling. With this hypothesis, the investigators selected patients with significant LV remodeling due to inferior myocardial infarction and without other lesions, therefore, similar degree and location of LV remodeling. In these selected patients with similar LV remodeling, PM dysfunction was associated with less MR and less leaflet tenting. These data indicate that PM dysfunction is not the primary cause of ischemic MR and that it may result in attenuated tethering and MR.
Although PM dysfunction does not generally cause ischemic MR, mitral valve prolapse with MR may occasionally develop in patients with ischemic heart disease or in animal models of disease.20)43-45) Such patients with myocardial infarction have leaflet prolapse with PM elongation or a short distance between the PM and the mitral annulus due to a hypercontractile LV. Further, the secondary MR responds to PM shortening or leaflet reduction.43)44) These observations, rather than disproving the tethering mechanism, actually confirm that the tethering distance from the PM tip to the mitral annulus is the final common pathway that determines the level of leaflet coaptation.
Annular dilatation is expected to worsen ischemic MR due to the structural characteristics of the mitral valve complex. The unclear point is whether it is a major determinant of the MR or not.
Mitral annular size is consistently larger in patients with ischemic MR compared to normal subjects.19)23)48-50) This suggests that annular dilatation is important in the mechanism of ischemic MR.51) However, significant overlap in the annular size between patients with and without ischemic MR has also been reported.52) The annular size was not significantly different before and after development of ischemic MR in an animal model of congestive heat failure.18) Further, persistent or recurrent ischemic MR can occur even following surgical annuloplasty.53-56) Therefore, it is not clear whether annular dilatation is a major determinant of ischemic MR or not.
The difficulty in characterizing the role of annular dilatation in the development of ischemic MR is came from that the annular dilatation and LV dilatation usually co-exist in patients with the MR. In an attempt to avoid this difficultly, we studied patients with lone atrial fibrillation, who often have left atrial dilatation without LV dilatation.57) Because the mitral annulus is positioned in between the left atrium and the LV, lone atrial fibrillation may cause isolated annular dilatation without LV dilatation. We have found that the annular dilatation in patients with lone atrial fibrillation was comparable to the annular dilatation in those with ischemic or idiopathic cardiomyopathy. Further, the annular dilatation in patients with lone atrial fibrillation was not associated with moderate to severe MR, while the same degree of the annular dilatation in those with cardiomyopathy was frequently associated with significant MR (Fig. 8).10) These data demonstrate that isolated annular dilatation, even with the same degree to patients with cardiomyopathy and significant MR, failed to cause significant MR and suggest that annular dilatation is not a major determinant of MR in the absence of leaflet tethering due to LV dilatation. However, annular dilatation may exert an effect on development of MR in the presence of leaflet tethering. For example, He et al.13) reported a greater increase in MR in response to annular dilatation in the presence of PM displacement in an in vitro model.
Loss of the physiological annular saddle shape may also contribute to the development of MR.58) Flachskampf, et al.59) and Gorman, et al.60) reported that the annulus flattens in ischemic or dilated ventricles, which may increase the distance between the commissural points and the PMs, potentially increasing tethering. Based on this concept, several groups have attempted to determine the effect of a modified ring shape on tethering and MR.
Multiple experimental and clinical studies seem to indicate that annular dilatation is not of critical importance to the development of ischemic MR.8-10)31)52) Regardless, annular size reduction by surgical annuloplasty, particularly by down sized ring annuloplasty, is usually effective in eliminating ischemic MR.61-64)
Development of ischemic MR due to leaflet tethering by outward displacement of the PMs can result from consumption of the physiological coaptation zone between the anterior and posterior leaflet (Fig. 9). Annular size reduction does not relieve the tethering and apical displacement of the leaflets but does shift the posterior annulus and leaflet anteriorly so that the consumed coaptation zone can be restored (Fig. 9).55) Thus, while surgical annuloplasty is effective in reversing ischemic MR, the procedure does not address tethering. Therefore, advanced tethering may result in persistent ischemic MR even after ring annuloplasty with or without down sizing.31)53-56)
As discussed above, leaflet tethering due to outward displacement of the PMs appears to be the main cause of the ischemic MR. Because the normal geometric relationship between the PMs and the chordal attachment at the leaflet is heterogeneous, leaflet tethering can vary within a single patient. One such example is the augmented tethering that occurs at the mid-portion of the anterior leaflet with basal or strut chordal insertion (Fig. 10).
Nesta et al.65)66) reported that anterior leaflet in the echocardiographic long axis view is convex toward the left atrium in normal subjects but is concave in patients with ischemic MR, suggesting that the middle portion of the anterior leaflet is more tethered compared to the leaflet tip. Based on these observations, Messas et al.67)68) reported that cutting of the basal chordae in an animal model of ischemic MR eliminated the MR by restoring the physiological convex configuration of the anterior leaflets toward the left atrium.
The degree and direction of outward displacement of the PMs can vary between patients, which suggest that leaflet tethering pattern may also vary between patients with ischemic MR. Kwan et al.69) reported that the medial side of the mitral leaflets is more apically displaced compared to lateral side of the leaflets in patients with ischemic MR due to inferior myocardial infarction, while the displacement was approximately symmetric when comparing the medial and lateral side in patients with MR due to dilated cardiomyopathy. The medial and lateral PM displacement was also asymmetric, with a predominance for the medial PM in patients with ischemic MR due to inferior myocardial infarction.70) By contrast, the PM displacement was symmetric in those with MR due to anterior myocardial infarction. It is, however, controversial whether asymmetric PM displacement causes asymmetric leaflet tenting or not. Watanabe et al.71) have observed approximately symmetric leaflet tenting in patients with inferior myocardial infarction with asymmetric PM displacement.
Surgical ring annuloplasty may also cause heterogeneous mitral leaflet tethering. Surgical ring annuloplasty, which reduces the antero-posterior dimension of the mitral annulus, displaces the posterior annulus anteriorly. However, the anterior annulus remains fixed at the aortic root, which can result in augmented tethering of the posterior leaflet while maintaining tethering of the anterior leaflet. This predominant tethering of the posterior mitral leaflet can be observed in the echocardiographic long axis view. Figure 10 shows usual leaflet tethering due to dilated cardiomyopathy that results in symmetric tethering of the anterior and posterior leaflets with increased angles between the leaflets and the line connecting the annuli. However, this angle becomes highly asymmetric following surgical annuloplasty for ischemic MR (Fig. 11). This asymmetric tethering of the posterior leaflet can be associated with persistent or recurrent ischemic MR after the surgery.72)73)
Further variability in mitral leaflet tethering may occur in the context of pathophysiology in patients with ischemic MR. A global and 3-dimensional approach may allow for comprehensive evaluation of the tethering of the whole leaflets and help guide surgery.74)
Augmented leaflet tethering due to the outward displacement of the PMs with LV remodeling appears to be a basic mechanism for ischemic MR. Further, annular dilatation and LV dysfunction likely contribute to the development of MR in the presence of augmented tethering. PM dysfunction per se does not usually cause ischemic MR and may occasionally attenuate tethering and MR. The concept of PM dyssynchrony is important and further study to separate effects of PM dyssynchrony from other factors are required. Although surgical annuloplasty is often effective in reversing ischemic MR, the frequency of patients with persistent or recurrent ischemic MR after surgical ring annuloplasty even with advanced down sizing suggests the need for approaches to address tethering. Finally, leaflet tethering in patients with ischemic MR can vary within a single patient and between patients, indicating the multiple and individualized approaches may be required to correct ischemic MR in affected patients.
Figures and Tables
Fig. 1
Apical displacement of the mitral leaflets in patients with Ischemic mitral regurgitation (MR). Normal mitral leaflets close at the annular level (yellow arrows). However, leaflets in patients with ischemic MR cannot reach the annular level and coapt at an apically displaced position (pink arrows).
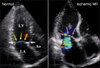
Fig. 2
Two superimposing and opposing forces on the leaflets to determine its closing position. Reduced closing force and augmented tethering force may result in apically displaced leaflets and mitral regurgitation (MR).
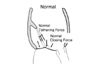
Fig. 3
Practical method to measure papillary muscle (PM) tethering distance. In the apical 4- and 2-chamber views, distances between PM tip and the contralateral anterior annulus express outward displacement of the lateral and medial PM relative to the anterior annulus, respectively.

Fig. 4
Potential mechanism for the higher incidence of ischemic mitral regurgitation in inferior versus anterior myocardial infarction (MI). Although, global LV remodeling in anterior MI may involve broader LV without causing major alterations in the mitral valve complex. In contrast, LV remodeling in response to inferior MI may involve less globally but may cause local and major alterations in the mitral valve complex.

Fig. 5
Phasic changes in the degree of ischemic mitral regurgitation (MR) during systole. MR is minimal in mid-systole (white arrows) with maximal closing pressure, suggesting that reduced closing force augment MR in early and late systole (pink arrows).
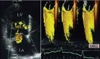
Fig. 6
Initial proposal of papillary muscle (PM) dysfunction to cause ischemic mitral regurgitation (MR). PM dysfunction with reduced PM longitudinal shortening may induce leaflet prolapse and ischemic MR.
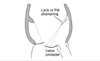
Fig.7
Two superimposing and opposing effects of papillary muscle (PM) dysfunction. Associated effects of PM dysfunction by remodeling of the adjacent wall may augment tethering and mitral regurgitation (MR). In contrast, direct effects of PM dysfunction reduced PM longitudinal shortening may reduce tethering and ischemic MR.
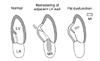
Fig. 8
Two representative patients with the same degree of mitral annular dilatation (pink arrows) and considerably different degrees of functional mitral regurgitation (MR). The left panel shows that annular dilatation in a patient with lone atrial fibrillation (Af) without left ventricular (LV) dilatation fails to cause leaflet tenting and significant MR. The right panel shows that the same degree of mitral annular dilatation in a patient with dilated cardiomyopathy (DCM) is associated with apically displaced leaflets and significant MR.
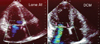
Fig. 9
Potential mechanism of beneficial surgical ring annuloplasty on ischemic mitral regurgitation (MR) despite that the surgery does not relieve tethering. The middle panel shows that development of the ischemic MR may be related to the consumption of physiological coaptation between the anterior and posterior leaflets. Surgical ring annuloplasty can potentially shift the posterior annulus and leaflet anteriorly so that the coaptation can be restored without rattenuating the tethering.

Fig. 10
Regional difference in tethering. Although apical displacement of leaflets involve whole anterior and posterior leaflets, the displacement is regionally augmented in the middle portion of the anterior leaflet (arrows).
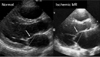
Fig. 11
A patient with recurrent functional mitral regurgitation (MR) following mitral annuloplasty and aortic valve replacement. The mitral annuli are connected with a white line. The LV is dilated, the anterior leaflet is tethered (white arrow), and the posterior leaflet is extremely tethered (yellow arrow), resulting in more posterior coaptation (pink arrow) than posterior annulus (thinner yellow arrow).
References
1. Tcheng JE, Jackman JD Jr, Nelson CL, Gardner LH, Smith LR, Rankin JS, Califf RM, Stack RS. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann Intern Med. 1992. 117:18–24.

2. Lehmann KG, Francis CK, Dodge HT. Mitral regurgitation in early myocardial infarction. Incidence, clinical detection, and prognostic implications TIMI Study Group. Ann Intern Med. 1992. 117:10–17.

3. Lamas GA, Mitchell GF, Flaker GC, Smith SC Jr, Gersh BJ, Basta L, Moye L, Braunwald E, Pfeffer MA. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation. 1997. 96:827–833.

4. Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001. 103:1759–1764.

5. Kisanuki A, Otsuji Y, Kuroiwa R, Murayama T, Matsushita R, Shibata K, Yutsudo T, Nakao S, Nomoto K, Tomari T, et al. Two-dimensional echocardiographic assessment of papillary muscle contractility in patients with prior myocardial infarction. J Am Coll Cardiol. 1993. 21:932–938.

6. Ogawa S, Hubbard FE, Mardelli TJ, Dreifus LS. Cross-sectional echocardiographic spectrum of papillary muscle dysfunction. Am Heart J. 1979. 97:312–321.

7. Godley RW, Wann LS, Rogers EW, Feigenbaum H, Weyman AE. Incomplete mitral leaflet closure in patients with papillary muscle dysfunction. Circulation. 1981. 63:565–571.

8. Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song JK, Guerrero JL, Vlahakes GJ, Levine RA. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997. 96:1999–2008.

9. Yiu SF, Enriquez-Sarano M, Tribouilloy C, Seward JB, Tajik AJ. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation. 2000. 102:1400–1406.

10. Otsuji Y, Kumanohoso T, Yoshifuku S, Matsukida K, Koriyama C, Kisanuki A, Minagoe S, Levine RA, Tei C. Isolated annular dilation does not usually cause important functional mitral regurgitation: comparison between patients with lone atrial fibrillation and those with idiopathic or ischemic cardiomyopathy. J Am Coll Cardiol. 2002. 39:1651–1656.

11. Kaul S, Spotnitz WD, Glasheen WP, Touchstone DA. Mechanism of ischemic mitral regurgitation. An experimental evaluation. Circulation. 1991. 84:2167–2180.

12. Dent JM, Spotnitz WD, Nolan SP, Jayaweera AR, Glasheen WP, Kaul S. Mechanism of mitral leaflet excursion. Am J Physiol. 1995. 269:H2100–H2108.

13. He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA. Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation. 1997. 96:1826–1834.

14. Otsuji Y, Handschumacher MD, Liel-Cohen N, Tanabe H, Jiang L, Schwammenthal E, Guerrero JL, Nicholls LA, Vlahakes GJ, Levine RA. Mechanism of ischemic mitral regurgitation with segmental left ventricular dysfunction: three-dimensional echocardiographic studies in models of acute and chronic progressive regurgitation. J Am Coll Cardiol. 2001. 37:641–648.

15. Llaneras MR, Nance ML, Streicher JT, Linden PL, Downing SW, Lima JA, Deac R, Edmunds LH Jr. Pathogenesis of ischemic mitral insufficiency. J Thorac Cardiovasc Surg. 1993. 105:439–442. discussion 442-3.

16. Gorman RC, McCaughan JS, Ratcliffe MB, Gupta KB, Streicher JT, Ferrari VA, St John-Sutton MG, Bogen DK, Edmunds LH Jr. Pathogenesis of acute ischemic mitral regurgitation in three dimensions. J Thorac Cardiovasc Surg. 1995. 109:684–693.

17. Komeda M, Glasson JR, Bolger AF, Daughters GT 2nd, Mac Isaac A, Oesterle SN, Ingels NB Jr, Miller DC. Geometric determinants of ischemic mitral regurgitation. Circulation. 1997. 96:9 Suppl. II-128–II-133.
18. Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Stein PD, Goldstein S. Mechanism of functional mitral regurgitation during acute myocardial ischemia. . J Am Coll Cardiol. 1992. 19:1101–1105.

19. Sabbah HN, Kono T, Rosman H, Jafri S, Stein PD, Goldstein S. Left ventricular shape: a factor in the etiology of functional mitral regurgitation in heart failure. Am Heart J. 1992. 123:961–966.

20. Izumi S, Miyatake K, Beppu S, Park YD, Nagata S, Kinoshita N, Sakakibara H, Nimura Y. Mechanism of mitral regurgitation in patients with myocardial infarction: a study using real-time two-dimensional Doppler flow imaging and echocardiography. Circulation. 1987. 76:777–785.

21. Leor J, Feinberg MS, Vered Z, Hod H, Kaplinsky E, Goldbourt U, Truman S, Motro M. Effect of thrombolytic therapy on the evolution of significant mitral regurgitation in patients with a first inferior myocardial infarction. J Am Coll Cardiol. 1993. 21:1661–1666.

22. Gorman JH, Gorman RC, Plappert T, Jackson BM, Hiramatsu Y, St John-Sutton MG, Edmunds LH Jr. Infarct size and location determine development of mitral regurgitation in the sheep model. J Thorac Cardiovasc Surg. 1988. 115:615–622.

23. Kumanohoso T, Otsuji Y, Yoshifuku S, Matsukida K, Koriyama C, Kisanuki A, Minagoe S, Levine RA, Tei C. Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. J Thorac Cardiovasc Surg. 2003. 125:135–143.

24. Liel-Cohen N, Guerrero JL, Otsuji Y, Handschumacher MD, Rudski LG, Hunziker PR, Tanabe H, Scherrer-Crosbie M, Sullivan S, Levine RA. Design of a new surgical approach for ventricular remodeling to relieve ischemic mitral regurgitation: insights from 3-dimensional echocardiography. Circulation. 2000. 101:2756–2763.

25. Hung J, Guerrero JL, Handschumacher MD, Supple G, Sullivan S, Levine RA. Reverse ventricular remodeling reduces ischemic mitral regurgitation: echo-guided device application in the beating heart. Circulation. 2002. 106:2594–2600.

26. Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg. 2002. 74:600–601.

27. Hvass U, Tapia M, Baron F, Pouzet B, Shafy A. Papillary muscle sling: a new functional approach to mitral repair in patients with ischemic left ventricular dysfunction and functional mitral regurgitation. Ann Thorac Surg. 2003. 75:809–811.

28. Dobre M, Koul B, Rojer A. Anatomic and physiologic correction of the restricted posterior mitral leaflet motion in chronic ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2000. 120:409–411.

29. Ueno T, Sakata R, Iguro Y, Nagata T, Otsuji Y, Tei C. A new surgical approach to reduce tethering in ischemic mitral regurgitation by relocation of separate heads of the posterior papillary muscle. Ann Thorac Surg. (in press).
30. Schwammenthal E, Chen C, Benning F, Block M, Breithardt G, Levine RA. Dynamics of mitral regurgitant flow and orifice area. Physiologic application of the proximal flow convergence method: clinical data and experimental testing. Circulation. 1994. 90:307–322.

31. Hung J, Otsuji Y, Handschumacher MD, Schwammenthal E, Levine RA. Mechanism of dynamic regurgitant orifice area variation in functional mitral regurgitation: physiologic insights from the proximal flow convergence technique. J Am Coll Cardiol. 1999. 33:538–545.

32. Breithardt OA, Sinha AM, Schwammenthal E, Bidaoui N, Markus KU, Franke A, Stellbrink C. Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J Am Coll Cardiol. 2003. 41:765–770.

33. Fukuda S, Grimm R, Song JM, Kihara T, Daimon M, Agler DA, Wilkoff BL, Natale A, Thomas JD, Shiota T. Electrical conduction disturbance effects on dynamic changes of functional mitral regurgitation. J Am Coll Cardiol. 2005. 12. 20. 46:2270–2276.

34. Burch GE, De Pasquale NP, Phillips JH. Clinical manifestations of papillary muscle dysfunction. Arch Intern Med. 1963. 112:112–117.

35. Burch GE, DePasquale NP, Phillips JH. The syndrome of papillary muscle dysfunction. Am Heart J. 1968. 75:399–415.

36. Hider CF, Taylor DE, Wade JD. The effect of papillary muscle damage on atrioventricular valve function in the left heart. Q J Exp Physiol Cogn Med Sci. 1965. 50:15–22.

37. Miller GE Jr, Kerth WJ, Gerbode F. Experimental papillary muscle infarction. J Thorac Cardiovasc Surg. 1968. 56:611–616.

38. Tsakiris AG, Rastelli GC, Amorim Dde S, Titus JL, Wood EH. Effect of experimental papillary muscle damage on mitral valve closure in intact anesthetized dogs. Mayo Clin Proc. 1970. 45:275–285.
39. Mittal AK, Langston M Jr, Cohn KE, Selzer A, Kerth WJ. Combined papillary muscle and left ventricular wall dysfunction as a cause of mitral regurgitation. An experimental study. Circulation. 1971. 44:174–180.

40. Matsuzaki M, Yonezawa F, Toma Y, Yonezawa F, Kohno M, Katayama K, Ozaki M, Kumada T, Kusukawa R. Experimental mitral regurgitation in ischemia-induced papillary muscle dysfunction. J Cardiol. 1988. 18:Suppl. 121–126.
41. Messas E, Guerrero JL, Handschumacher MD, Chow CM, Sullivan S, Schwammenthal E, Levine RA. Paradoxic decrease in ischemic mitral regurgitation with papillary muscle dysfunction: insights from three-dimensional and contrast echocardiography with strain rate measurement. Circulation. 2001. 104:1952–1957.

42. Uemura T, Otsuji Y, Nakashiki K, Yoshifuku S, Maki Y, Yu B, Mizukami N, Kuwahara E, Hamasaki S, Biro S, Kisanuki A, Minagoe S, Levine RA, Tei C. Papillary muscle dysfunction attenuates ischemic mitral regurgitation in patients with localized basal inferior left ventricular remodeling: insights from tissue Doppler strain imaging. J Am Coll Cardiol. (in press).
43. Fundaro P, Pocar M, Moneta A, Donatelli F, Grossi A. Posterior mitral valve restoration for ischemic regurgitation. Ann Thorac Surg. 2004. 77:729–730.

44. Jouan J, Tapia M, C Cook R, Lansac E, Acar C. Ischemic mitral valve prolapse: mechanisms and implications for valve repair. Eur J Cardiothorac Surg. 2004. 26:1112–1117.

45. Tei C, Sakamaki T, Shah PM, Meerbaum S, Kondo S, Shimoura K, Corday E. Mitral valve prolapse in short-term experimental coronary occlusion: a possible mechanism of ischemic mitral regurgitation. Circulation. 1983. 68:183–189.

46. Kanzaki H, Bazaz R, Schwartzman D, Dohi K, Sade LE, Gorcsan J 3rd. A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy: insights from mechanical activation strain mapping. J Am Coll Cardiol. 2004. 44:1619–1625.

47. Ypenburg C, Lancellotti P, Tops LF, Bleeker GB, Holman ER, Piérard LA, Schalij MJ, Bax JJ. Acute effects of initiation and withdrawal of cardiac resynchronization therapy on papillary muscle dyssynchrony and mitral regurgitation. J Am Coll Cardiol. 2007. 50:2071–2077.

48. Ahmad RM, Gillinov AM, McCarthy PM, Blackstone EH, Apperson-Hansen C, Qin JX, Agler D, Shiota T, Cosgrove DM. Annular geometry and motion in human ischemic mitral regurgitation: novel assessment with three-dimensional echocardiography and computer reconstruction. Ann Thorac Surg. 2004. 78:2063–2068.

49. Tibayan FA, Rodriguez F, Zasio MK, Bailey L, Liang D, Daughters GT, Langer F, Ingels NB Jr, Miller DC. Geometric distortions of the mitral valvular-ventricular complex in chronic ischemic mitral regurgitation. Circulation. 2003. 108:Suppl 1. II116–II121.

50. Green GR, Dagum P, Glasson JR, Daughters GT, Bolger AF, Foppiano LE, Berry GJ, Ingels NB Jr, Miller DC. Mitral annular dilatation and papillary muscle dislocation without mitral regurgitation in sheep. Circulation. 1999. 100(19):Suppl. II95–II102.

51. Boltwood CM, Tei C, Wong M, Shah PM. Quantitative echocardiography of the mitral complex in dilated cardiomyopathy: the mechanism of functional mitral regurgitation. Circulation. 1983. 68:498–508.

52. Chandraratna PAN, Aronow WS. Mitral valve ring in normal vs dilated left ventricle: cross-sectional echocardiographic study. Chest. 1981. 79:151–154.

53. Calafiore AM, Gallina S, Di Mauro M, Gaeta F, Iaco AL, D'Alessandro S, Mazzei V, Di Giammarco G. Mitral valve procedure in dilated cardiomyopathy: repair or replacement? Ann Thorac Surg. 2001. 71:1146–1152.

54. Tahta SA, Oury JH, Maxwell JM, Hiro SP, Duran CM. Outcome after mitral valve repair for functional ischemic mitral regurgitation. J Heart Valve Dis. 2002. 11:11–18.
55. McGee EC Jr, Gillinov AM, Blackstone EH, Rajeswaran J, Cohen G, Najam F, Shiota T, Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004. 128:916–924.

56. Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen BA, Duran CM, Levine RA. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation. 2004. 110(11):Suppl 1. II85–II90.

57. Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA, Weyman AE. Atrial enlargement as a consequence of atrial fibrillation: a prospective echocardiographic study. Circulation. 1990. 82:792–797.

58. Levine RA, Handschumacher MD, Sanfilippo AJ, Hagege AA, Harrigan P, Marshall JE, Weyman AE. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989. 80:589–598.

59. Flachskampf FA, Chandra S, Gaddipatti A, Levine RA, Weyman AE, Ameling W, Hanrath P, Thomas JD. Analysis of shape and motion of the mitral annulus in subjects with and without cardiomyopathy by echocardiographic 3-dimensional reconstruction. J Am Soc Echocardiogr. 2000. 13:277–287.

60. Gorman JH 3rd, Jackson BM, Enomoto Y, Gorman RC. The effect of regional ischemia on mitral valve annular saddle shape. Ann Thorac Surg. 2004. 77:544–548.

61. Bolling SF, Pagani FD, Deeb GM, Bach DS. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg. 1998. 115:381–386. discussion 387-8.

62. Bach DS, Bolling SF. Improvement following correction of secondary mitral regurgitation in end-stage cardiomyopathy with mitral annuloplasty. Am J Cardiol. 1996. 78:966–969.

63. Bach DS, Bolling SF. Early improvement in congestive heart failure after correction of secondary mitral regurgitation in end-stage cardiomyopathy. Am Heart J. 1995. 129:1165–1170.

64. Bax JJ, Braun J, Somer ST, Klautz R, Holman ER, Versteegh MI, Boersma E, Schalij MJ, van der Wall EE, Dion RA. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation. 2004. 110(11):Suppl 1. II103–II108.

65. Weyman AE. Weyman AE, editor. Part 2 Clinical applications, 17 left ventricular inflow tract I: the mitral valve. Principles and Practice of Echocardiography. 1994. Philadelphia: Lea & Febiger;457.
66. Nesta F, Otsuji Y, Handschumacher MD, Messas E, Leavitt M, Carpentier A, Levine RA, Hung J. Leaflet concavity: a rapid visual clue to the presence and mechanism of functional mitral regurgitation. J Am Soc Echocardiogr. 2003. 16:1301–1308.

67. Messas E, Guerrero JL, Handschumacher MD, Conrad C, Chow CM, Sullivan S, Yoganathan AP, Levine RA. Chordal cutting: a new therapeutic approach for ischemic mitral regurgitation. Circulation. 2001. 104:1958–1963.
68. Messas E, Pouzet B, Touchot B, Guerrero JL, Vlahakes GJ, Desnos M, Menasche P, Hagege A, Levine RA. Efficacy of chordal cutting to relieve chronic persistent ischemic mitral regurgitation. Circulation. 2003. 108:Suppl 1. II111–II115.

69. Kwan J, Shiota T, Agler DA, Popovic ZB, Qin JX, Gillinov MA, Stewart WJ, Cosgrove DM, McCarthy PM, Thomas JD. Real-time three-dimensional echocardiography study. Geometric differences of the mitral apparatus between ischemic and dilated cardiomyopathy with significant mitral regursgitation: real-time three-dimensional echocardiography study. Circulation. 2003. 107:1135–1140.

70. Zhang H, Otsuji Y, Uemura T, Kumanohoso T, Yu B, Zhou X, Levine RA, Kisanuki A. Different mechanisms of ischemic mitral regurgitation in patients with anterior and inferior myocardial infarction. Circulation. 2003. 108:suppl IV. IV-432.
71. Watanabe N, Ogasawara Y, Yamaura Y, Yamamoto K, Wada N, Kawamoto T, Toyota E, Akasaka T, Yoshida K. Geometric differences of the mitral valve tenting between anterior and inferior myocardial infarction with significant ischemic mitral regurgitation: quantitation by novel software system with transthoracic real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2006. 19:71–75.

72. Zhu F, Otsuji Y, Yotsumoto G, Yuasa T, Ueno T, Yu B, Koriyama C, Hamasaki S, Biro S, Kisanuki A, Minagoe S, Levine RA, Sakata R, Tei C. Mechanism of persistent ischemic mitral regurgitation following annuloplasty: importance of augmented posterior mitral leaflet tethering. Circulation. 2005. 112:suppl I. I-396–I-401.
73. Kuwahara E, Otsuji Y, Iguro Y, Ueno T, Zhu F, Mizukami N, Kubota K, Nakashiki K, Yuasa T, Yu B, Uemura T, Takasaki K, Miyata M, Hamasaki S, Kisanuki A, Levine RA, Sakata R, Tei C. Mechanism of Recurrent/Persistent Ischemic/Functional Mitral Regurgitation in the Chronic Phase Following Surgical Annuloplasty: Importance of Augmented Posterior Leaflet Tethering. Circulation. 2006. 114:Suppl I. I-529–I-534.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download