Abstract
Connexins (Cx) are membrane proteins and monomers for forming gap junction (GJ) channels. Cx46 and Cx50 are also known to function as conductive hemichannels. As part of an ongoing effort to find GJ-specific blocker(s), endocrine disruptors were used to examine their effect on Cx46 hemichannels expressed in Xenopus oocytes. Voltage-dependent gating of Cx46 hemichannels was characterized by slowly activating outward currents and relatively fast inward tail currents. Bisphenol A (BPA, 10 nM) reduced outward currents of Cx46 hemichannels up to ~18% of control, and its effect was reversible (n=5). 4-tert-Octylphenol (OP, 1 µM) reversibly reduced outward hemichannel currents up to ~28% (n=4). However, overall shapes of Cx46 hemichannel current traces (outward and inward currents) were not changed by these drugs. These results suggest that BPA and OP are likely to occupy the pore of Cx46 hemichannels and thus obstruct the ionic fluxes. This finding provides that BPA and OP are potential candidates for GJ channel blockers.
Gap junctions (GJs) are intercellular channels that provide the direct pathways for transmission of ions, second messengers, and cellular metabolites between adjacent cells. GJ channels are widely observed in both excitable and most non-excitable tissues in all animals [1]. Since first connexin (Cx) gene (connexin32, Cx32) has been identified from rat liver tissues [2], more than 22 members of the connexin gene family have been identified in vertebrates to date [3]. Cx is referred to by its predicted molecular weight in kilo dalton (kDa) [4], although an alternative nomenclature has been proposed [5]. Connexon (hemichannel) is composed of six Cx subunits. Two connexons in series forms a complete GJ channel. Except Cx31.1 [6] and Cx33 [7], all other connexins form functional intercellular channels when examined in the Xenopus oocytes expression system.
Both Xenopus oocyte and cell line expression systems are used to measure the ionic conductance mediated by GJ channels [8,9]. While macroscopic currents are obtained from GJ channels expressed in Xenopus oocytes, microscopic currents (also called single channel conductance) are acquired from GJ channels expressed in cell lines. Although single channel recordings provide more detailed information regarding channel functions, the Xenopus oocyte expression system is still valuable in screening functional GJ channels in a large scale, and in accessing the kinetics of channel functions. Since it has been reported that Cx46 form functional hemichannels in Xenopus oocytes [10] and these hemichannels share most of their properties including voltage dependence and channel gating with their parental GJ channels [11], Cx46 hemichannels have been widely used to characterize the biophysical properties of GJ channels.
Endocrine disruptors are chemicals that function like endogenous hormones and disturb the normal biological functions of the endocrine system. In general, endocrine disruptors include a wide range of chemical compounds such as pollutants, industrial by-products, pesticides, and even compounds used in consumer products [12]. In fact, ovaries and testes are the sites where the most connexin types are expressed. In the ovarian follicle, multiple connexins including Cx26, Cx30.3, Cx32, Cx37, Cx40, Cx43 and Cx45 are expressed [13,14]. Cx37 and Cx43 form GJ channels between the oocyte and granulosa cells, while other connexins form channels between granulosa cells. Likewise, expression of multiple connexins has been observed in testis [15]. Cx26, Cx32, Cx33, and Cx43 form intercellular channels both between Sertoli cells and between gamete and Sertoli cell. Therefore, endocrine disruptors are next candidates to be screened for GJ blockers. Although many studies have shown that the effects of endocrine disruptors on different experimental conditions diverse (reviewed in [16]) the studies investigating the effects of those drugs on ion channels are particularly interesting. Among them, bisphenol A (BPA) has been extensively used for examining its effect on ion channels. For examples, BPA activates BK (KCa1.1) channel expressed in AD 293 cells in subunit-specific manner [17] while BPA inhibits voltage-gated sodium channel (hNav1.5) expressed in HEK 293 cells [18]. BPA also inhibits voltage-activated Ca2+ channels expressed in different cell types by directly interacting with channel protein [19]. It has also been reported that the effects of nonylphenol (NP) on voltage-gated K+ channels and L-type Ca2+ channels in GH3 rat pituitary cells [20] are biphasic; i.e., NP decreases K+ currents and increases Ca2+ currents in low concentrations while NP shows opposite effects in higher concentrations.
There is a growing need to identify GJ channel blockers. Although many pharmacological agents have been used to evaluate their potency as channel blockers, the outcomes have not been satisfied to meet the potency and specificity of conventional ion channel blockers [21,22,23,24]. Furthermore, it is difficult to determine the fast kinetics of inhibition if GJ channels are used.
To examine the potency of endocrine disruptors as GJ channel blockers, Cx46 hemichannels expressed in Xenopus oocytes were used instead of using the Cx46 GJ channels. The outward currents elicited by the application of depolarizing potentials were measured to determine if currents were changed after bath application of those chemicals.
Complementary RNA (cRNA) was synthesized from a linearized plasmid template containing connexin 46 coding sequences using 'mMESSAGE mMACHINE T7 kit' (Life Technologies, Carlsbad, CA, USA) according to the manufacturer's protocol. For the preparation of oocytes and cRNA injection into Xenopus oocytes the procedures described in previous reports [25,26] were used. Approximately 50 nl of 1 ng/nl RNA was co-injected into Xenopus oocytes with 0.3 pmol/nl of an antisense phosphorotioate oligonucleotide complimentary to Xenopus Cx38 [26]. After RNA injection, oocytes were kept in a bath solution containing 88 mM NaCl, 1 mM KCl, 5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 0.1% glucose, and 2.5 mM pyruvate (pH 7.6).
Six common endocrine disruptors were used: 4-tert-octylphenol (OP, M.W. 206.32), nonylphenol (NP, M.W. 220.35), bisphenol A (BPA, M.W. 228.29), benzo[a]pyrene (BP, M.W. 252.31), mono-ethylhexyl phthalate (MEHP, M.W. 278.34), and di-ethylhexyl phthalate (DEHP, M.W. 390.56). OP, BP, MEHP and DEHP were purchased from Tokyo Chemical Industry (Tokyo, Japan). NP and BPA were purchased from Sigma-Aldrich (St. Louis, MO, USA). All stock solutions were made in dimethyl sulfoxide (DMSO) except DEHP (in ethanol). Final working solutions were freshly made in bath solution before use.
Macroscopic current recordings from single Xenopus oocytes were obtained by employing a two-electrode voltage clamp technique. Both voltage and current microelectrodes were filled with 1 M KCl. The outward currents elicited by the application of depolarizing potential were measured with or without each endocrine disruptor. Endocrine disruptors were perfused by gravity. Macroscopic currents were acquired at 200 Hz using OC-725C Oocyte voltage clamp (Warner Instrument, Hamden, CT, USA) and iwx118 digidata interface and LabScribe Software (iWork/CB Sciences, Dover, NH, USA). Peak current was determined by the difference between maximal and minimal current values during a 10-second pulse. All current values were presented by means±standard deviations. The data were analyzed by Student t-test with p<0.05 considered to be statistically significant. Microcal Origin 8.0 (OriginLab Corporation, Northampton, MA, USA) and CorelDRAW 9.0 (Corel Corporation, Ottawa, Canada) were used for data analysis and illustration.
To examine the expression of Cx46 hemichannels in Xenopus oocyte, macroscopic currents of Cx46 hemichannels were elicited by the application of depolarizing potentials (Fig. 1A). Currents of Cx46 hemichannels at -70 mV holding potential were near 0. When stepping voltage from a holding potential to a depolarizing potential at +30 mV for 10 seconds, Cx46 hemichannels showed slowly activating outward currents. It has been reported that outward currents by depolarizing potentials indicate the opening of Cx46 hemichannels [11,27]. Repolarization to -70 mV holding potential produced relatively fast inward tail currents. The closure of Cx46 hemichannels is represented by inward tail currents [11,27]. The optimized pulse protocol (-70 mV holding potential, +30 mV depolarizing potential for 10 seconds, and total recoding duration of 40 seconds) was used throughout the experiments. Un-injected Xenopus oocytes were used as a control group. When applying same pulse protocol, control oocytes did not show any slowly activating outward and relatively fast inward currents as those of Cx46 cRNA injected oocytes (Fig. 1B).
To test the effect of endocrine disruptors on hemichannels, at least triple phases (control-drug-wash) of a record obtained from a single Xenopus oocyte were required. To ensure that currents were stable for entire recording, triple sweeps were used for each phase. To minimize both inter-sweep and inter-phase differences, currents at each sweep were recorded immediately after the perfusion with new bath solution. Using this recording scheme, macroscopic currents were measured from a single Xenopus oocyte expressing Cx46 hemichannels (Fig. 1C). Most peak currents were quite stable for entire recording periods indicated by the dotted line. Since the inward tail currents always returned to zero level within a few seconds, current traces about 25 seconds before the next sweep were omitted for data illustration.
Common endocrine disruptors were tested to determine whether those drugs affected Cx46 hemichannel currents. Drug studies using Cx46 hemichannels expressed in Xenopus oocytes are not currently available. The concentrations of endocrine disruptors used in this study were determined based on the results of the previous studies [28,29,30,31,32,33]. Bisphenol A (BPA) was first examined to determine whether it has the potency to affect Cx46 hemichannel currents (Fig. 2A). In the control phase (initial three sweeps), the average peak current of the three sweeps was 4.75±0.48 µA. After applying 10 nM BPA (middle three sweeps), the average peak current was reduced to 3.45±0.05 µA (27.36% reduction). However, voltage dependence of Cx46 hemichannel (outward and inward current traces) was not changed by BPA. The average peak current (4.64±0.40 µA) in the last three sweeps (wash phase) was returned to the control level (indicated by a dotted line), indicating that the effect of BPA was reversible. To analyze all data obtained from the five independent experiments, a normalized peak current of each phase was determined by the ratio of average peak currents of the drug (or wash) phase to those of the control phase (Fig. 2B). Normalized peak currents with BPA treatment were 0.82±0.10 (n=5) and this inhibitory effect (~18% reduction of control) was statistically significant (p<0.05). Normalized peak current of the wash phase were 1.06±0.15 (n=5) suggesting that the effect of BPA on Cx46 hemichannel currents is reversible.
The effect of 4-tert-Octylphenol (OP) on Cx46 hemichannel currents is shown in Fig. 3A. The average peak current in the control phase was 1.60±0.13 µA. This current was inhibited by the treatment of 1 µM OP and thus the average peak current was reduced to 1.06±0.02 µA. The reduction rate for average peak current was 33.72%. Reduced peak currents were fully recovered by elimination of the drug. The average peak current in the wash phase was 1.52±0.06 µA, indicating that the effect of OP is as reversible as BPA. Overall shapes for outward and inward currents were not affected by 1 µM OP. Normalized peak currents from the four independent experiments are shown in Fig. 3B. Normalized peak currents inhibited by OP were 0.72±0.10 (n=4) and the reduction rate of the current (~28%) was statistically significant (p<0.05). The inhibitory effect of OP was also reversible (0.99±0.07, n=4).
Other endocrine disruptors including nonylphenol (NP), benzo[a]pyrene (BP), di-ethylhexyl phthalate (DEHP), and mono-ethylhexyl phthalate (MEHP) were also tested. The concentrations of each endocrine disruptor were 1 µM for NP, 10 µM for DEHP and MEHP, 100 µM for BP, respectively. None of them appeared to block Cx46 hemichannel currents (Fig. 4). The average peak currents in control phase of each drug experiments were 2.61±0.01 µA for NP, 2.07±0.01 µA for BP, 1.26±0.11 µA for MEHP, and 1.86±0.03 µA for DEHP, respectively (Fig. 4A). These average peak currents in control phases were quite stable during the entire periods of recordings indicated by dotted lines. The average peak currents in drug phase were 2.55±0.04 µA for NP, 2.03±0.03 µA for BP, 1.14±0.01 µA for MEHP, and 1.74±0.01 µA for DEHP, respectively. The average peak currents in wash phase were 2.39±0.06 µA for NP, 1.92±0.02 µA for BP, 1.13±0.03 µA for MEHP, and 1.71±0.01 µA for DEHP, respectively. In addition overall shapes for outward and inward currents of Cx46 hemichannels were not affected by these drugs. These results indicate that these drugs do not show any inhibitory effects on Cx46 hemichannel currents. Normalized peak currents from the independent experiments are shown in Fig. 4B. Normalized peak currents with drug treatments were 0.99±0.05 for NP (n=3), 1.03±0.05 for BP (n=3), 0.98±0.07 for MEHP (n=4), 1.01±0.06 for DEHP (n=3), respectively. Normalized peak currents from wash phase of each drug experiment were 0.95±0.05 for NP (n=3), 0.99±0.05 for BP (n=3), 0.96±0.05 for MEHP (n=4), 0.99±0.06 for DEHP (n=3), respectively. Any of those values were not significantly different from those of control indicating no inhibitory effects of these drugs on Cx46 hemichannel currents.
Endocrine disruptors used in this study were selected to screen for their potency as channel blockers. Since the peak currents of Cx46 hemichannels were used as an indicator of drug effect, the stability of currents for the duration of the entire recording was very important. It has been reported that there are mechanosensitive channels in Xenopus oocytes [34,35,36]. These endogenous channels are often activated by the stream of solution. The current contribution from endogenous channels to the outward currents of exogenous channels is often resulted in the appearance of fluctuating peak currents [37,38]. To eliminate this unrelated effect, we applied same mechanical stimuli to each sweep. Currents at each sweep were recorded immediately after the perfusion with new bath solution to mimic mechanical stimuli. The protocol established in this study was enough to overcome this stability issue.
BPA and OP showed their potency to reduce the peak currents of Cx46 hemichannels in this study. Although the inhibitory effect on hemichannel currents are induced by the extracellular (bath) application of drugs, hydrophobic BPA and OP are likely membrane-permeable and thus both extracellular and intracellular domains of Cx46 hemichannels should be equally considered as the binding sites for drugs. In this study, drug perfusion took about two minutes to completely replace the old bath solution. An additional few minutes were also required for inter-sweep perfusion. Therefore, it is likely that drugs used in this study can access to the intracellular domains of Cx46 hemichannels. Further study using mutant Cx46 hemichannels generated by either domain swap or site-directed mutagenesis techniques is necessary to define the amino acid residues of the binding site.
Endocrine disruptors used in this study have molecular weights ranging from 206.32 Da. for OP to 390.56 Da. for DEHP. Based on the results from both studies [9,39], the pore diameter of Cx32 GJ channel is between 12 and 14 angstrom whereas that of Cx43 GJ channel is 15 angstrom. This approximation for the pore diameter of GJ channel in general is confirmed by the crystal structure of Cx26 GJ channel [40]. This Cx26 channel structure provides that the amino-terminal regions of the six subunits make a funnel to restrict the pore diameter to 14 angstrom. Furthermore, the Cx26 crystal structure gives us more detailed information that the residues of the amino acids located on both the amino-terminal regions and the first transmembrane domains of subunits would interact to form the circular hydrogen bond network. Although any experimental data is currently unavailable, it is reasonable to put the pore diameter of Cx46 hemichannel within these ranges. PEG 300 (polyethylene glycol M.W. 300) is the upper limit to be passed through Cx32 GJ channel [9]. This means that the molecules having a smaller molecular weight than that of PEG 300 could be able to occlude the pore regions of the hemichannels. This approximation is supported by the results from this study. The peak currents of Cx46 hemichannels were reduced by only BPA and OP. The other endocrine disruptors did not show inhibitory effects on Cx46 hemichannel currents. In fact, BPA and OP have the smallest molecular weights among endocrine disruptors tested in this study. The presence of either OP or BPA in the pore region of Cx46 hemichannels is resulted in the obstruction of ionic flow. It was determined that all other endocrine disruptors including BP, MEHP, and DEHP were not able to occupy hemichannel pores based on the result that those chemicals did not affect the peak currents of Cx46 hemichannels. However, NP is categorized into a group including OP and BPA due to its similar molecular weight, although its inhibitory effect on currents was not observed in this study. The molecular weight of NP (220.36) is slightly smaller than that of BPA (228.29). Although the precise molecular structures of both endocrine disruptors in solution are not available, it is assumed that a long side backbone of NP might not be fitted to the pore of Cx46 hemichannels, and thus NP did not show the blocking effect on Cx46 hemichannels.
The effect of BPA on Cx46 hemichannel currents is particularly interesting for its concentration. Extensive studies have shown that the health effect of BPA on human and laboratory animals occurs in relatively low concentrations compared with those of other known endocrine disruptors [41]. Likewise, the effect of BPA on gap junction-mediated intercellular communication (GJIC) in low concentration has also been reported [42]. They showed that a 75% reduction of GJIC was observed within one hour when rat epithelium-derive BICR-M1Rk cells (Cx43 is a major GJ channel in this cell) were cultured in a medium containing 400 nM of BPA. As mentioned in previous section, the assumption is that all three types of GJ channels (Cx32, Cx43, and Cx46) are pretty much similar in pore dimension. The same assumption is equally applicable to the concentration issue. The concentration of BPA required for blocking hemichannels (Cx46 in this study) is lower than that for GJ channels (Cx43 in their study). BPA is immediately accessible to Cx46 hemichannel pores through their extracellular surface. In a GJ channel formed by Cx43, the extracellular site is not available because two Cx43 hemichannels join together to form a complete Cx43 GJ channel. The only site for BPA to block GJIC of Cx43 GJ channels is the intracellar surface of the channel pore. Hydrophobic BPA is likely membrane-permeable. It appears that higher BPA concentrations will be required for it to be localized to the intracellular pore region of Cx43 GJ channels. Therefore, it is favorable that the concentration required for affecting hemichannel currents is somewhat lower than that for GJ channel currents.
It was observed that higher concentration of OP (1 µM) was required for blocking Cx46 hemichannels compared with that of BPA (10 nM). Although the reduction rate of OP is a little higher than that of BPA, a hundred-fold difference in concentration brings about another issue to be explained. OP has a ring structure, whereas BPA has two rings. If the structure of BPA with two phenol rings is well fitted to Cx46 hemichannel pores, BPA will exert its blocking effect on hemichannel currents. Two or more ring structures from OP molecules will be required to block the hemichannel pores if one is not enough to properly occupy the channel pores. More OP molecules are therefore required. This assumption will be verified if the experimental data showing dose-dependents effect of these drugs are available. Therefore, it is interesting to know whether the inhibitory effects of both BPA and OP on Cx46 hemichannel currents are dose-dependent. Single hemichannel recordings employing patch clamp techniques with different concentrations will define the precise concentration-dependent effect of endocrine disruptors. The experiments using mutant Cx46 hemichannels will also further characterize BPA and OP as GJ blockers in more detail.
The molecular weights and structural compositions of drugs tested in this study are only known information. The interpretation of the results from this study is based on the idea that the fitting of drugs to the pore regions of Cx46 hemichannels is important for blocking the hemichannel currents; i.e., the drugs have to satisfy the structural requirements to be effective on hemichannels. This idea is strongly supported by a recent study [19]. This study provides that BPA directly interacts with the extracellular binding region of voltage-activated Ca2+ channels and this interaction varies depending on both the moieties of the carbon atom between two aromatic rings of BPA and the angulated orientation of the two aromatic rings. Furthermore, other study [18] showing the hydrophobic interaction between two phenol rings of BPA molecule and the biding sites of hNav 1.5 (the human cardiac sodium channel) further supports current interpretation. It is interesting to know whether the hydrophobic residues of the amino acids resided at both the amino-terminal regions and the first transmembrane domains of Cx46 hemichannel subunits make the hydrophobic interactions with BPA or OP. This will be verified by using mutant Cx46 hemichannels with different site of mutagenesis.
In summary, both BPA and OP show their inhibitory effects on the outward currents of Cx46 hemichannels expressed in Xenopus oocytes. Based on the present results it is likely that BPA and OP occupy the pore regions of Cx46 hemichannels and thus retard current flows. This electrophysiological finding suggests that both BPA and OP are potential candidates for GJ channel blockers. Singlechannel studies of GJ channels as well as conductive hemichannels promise to provide more a detailed mechanistic picture of the effects of endocrine disruptors on GJ channels.
ACKNOWLEDGEMENTS
This work was supported by National Research Foundation of Korea Grant funded by the Korean Government (NRF-2009-0077630).
References
1. Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001; 34:325–472. PMID: 11838236.
2. Paul DL. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986; 103:123–134. PMID: 3013898.

3. Söhl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004; 62:228–232. PMID: 15094343.
4. Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987; 105:2621–2629. PMID: 2826492.

5. Kumar NM, Friend DS, Gilula NB. Synthesis and assembly of human beta 1 gap junctions in BHK cells by DNA transfection with the human beta 1 cDNA. J Cell Sci. 1995; 108:3725–3734. PMID: 8719879.

6. Hennemann H, Dahl E, White JB, Schwarz HJ, Lalley PA, Chang S, Nicholson BJ, Willecke K. Two gap junction genes, connexin 31.1 and 30.3, are closely linked on mouse chromosome 4 and preferentially expressed in skin. J Biol Chem. 1992; 267:17225–17233. PMID: 1512260.

7. Chang M, Werner R, Dahl G. A role for an inhibitory connexin in testis? Dev Biol. 1996; 175:50–56. PMID: 8608868.

8. Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994; 368:348–351. PMID: 8127371.
9. Oh S, Ri Y, Bennett MV, Trexler EB, Verselis VK, Bargiello TA. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron. 1997; 19:927–938. PMID: 9354338.

10. Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991; 115:1077–1089. PMID: 1659572.

11. Trexler EB, Bennett MV, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci U S A. 1996; 93:5836–5841. PMID: 8650179.

12. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009; 30:293–342. PMID: 19502515.

13. Wright CS, Becker DL, Lin JS, Warner AE, Hardy K. Stage-specific and differential expression of gap junctions in the mouse ovary: connexin-specific roles in follicular regulation. Reproduction. 2001; 121:77–88. PMID: 11226030.

14. Grazul-Bilska AT, Reynolds LP, Redmer DA. Gap junctions in the ovaries. Biol Reprod. 1997; 57:947–957. PMID: 9369157.
15. Risley MS. Connexin gene expression in seminiferous tubules of the Sprague-Dawley rat. Biol Reprod. 2000; 62:748–754. PMID: 10684819.
16. Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012; 33:378–455. PMID: 22419778.

17. Rottgen TS, Fancher IS, Asano S, Widlanski TS, Dick GM. Bisphenol A activates BK channels through effects on α and β1 subunits. Channels (Austin). 2014; 8:249–257.

18. O'Reilly AO, Eberhardt E, Weidner C, Alzheimer C, Wallace BA, Lampert A. Bisphenol A binds to the local anesthetic receptor site to block the human cardiac sodium channel. PLoS One. 2012; 7:e41667. PMID: 22848561.
19. Deutschmann A, Hans M, Meyer R, Häberlein H, Swandulla D. Bisphenol A inhibits voltage-activated Ca2+ channels in vitro: mechanisms and structural requirements. Mol Pharmacol. 2013; 83:501–511. PMID: 23197648.
20. Gao Q, Zhu T, Guo F, Huang S, Hu H, Feng R, Hao L. Nonylphenol, an environmental estrogen, affects voltage-gated K+ currents and L-type Ca2+ currents in a non-monotonic manner in GH3 pituitary cells. Toxicol Lett. 2013; 218:137–143. PMID: 23376477.
21. Spray DC, Rozental R, Srinivas M. Prospects for rational development of pharmacological gap junction channel blockers. Curr Drug Targets. 2002; 3:455–464. PMID: 12448697.
22. Boger DL, Patterson JE, Guan X, Cravatt BF, Lerner RA, Gilula NB. Chemical requirements for inhibition of gap junction communication by the biologically active lipid oleamide. Proc Natl Acad Sci U S A. 1998; 95:4810–4815. PMID: 9560184.

23. Srinivas M, Hopperstad MG, Spray DC. Quinine blocks specific gap junction channel subtypes. Proc Natl Acad Sci U S A. 2001; 98:10942–10947. PMID: 11535816.

24. Kim DY, Jung CS. Gap junction contributions to the goldfish electroretinogram at the photopic illumination level. Korean J Physiol Pharmacol. 2012; 16:219–224. PMID: 22802705.

25. Oh S, Abrams CK, Verselis VK, Bargiello TA. Stoichiometry of transjunctional voltage-gating polarity reversal by a negative charge substitution in the amino terminus of a connexin32 chimera. J Gen Physiol. 2000; 116:13–31. PMID: 10871637.

26. Rubin JB, Verselis VK, Bennett MV, Bargiello TA. Molecular analysis of voltage dependence of heterotypic gap junctions formed by connexins 26 and 32. Biophys J. 1992; 62:183–193. PMID: 1376166.

27. Trexler EB, Bukauskas FF, Kronengold J, Bargiello TA, Verselis VK. The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys J. 2000; 79:3036–3051. PMID: 11106610.

28. Derouiche S, Warnier M, Mariot P, Gosset P, Mauroy B, Bonnal JL, Slomianny C, Delcourt P, Prevarskaya N, Roudbaraki M. Bisphenol A stimulates human prostate cancer cell migration via remodelling of calcium signalling. Springerplus. 2013; 2:54. PMID: 23450760.

29. Chung JY, Kim JY, Kim YJ, Jung SJ, Park JE, Lee SG, Kim JT, Oh S, Lee CJ, Yoon YD, Yoo YH, Kim JM. Cellular defense mechanisms against benzo[a]pyrene in testicular Leydig cells: implications of p53, aryl-hydrocarbon receptor, and cytochrome P450 1A1 status. Endocrinology. 2007; 148:6134–6144. PMID: 17884947.

30. Kotula-Balak M, Chojnacka K, Hejmej A, Galas J, Satola M, Bilinska B. Does 4-tert-octylphenol affect estrogen signaling pathways in bank vole Leydig cells and tumor mouse Leydig cells in vitro? Reprod Toxicol. 2013; 39:6–16. PMID: 23557686.

31. Liu PS, Liu GH, Chao WL. Effects of nonylphenol on the calcium signal and catecholamine secretion coupled with nicotinic acetylcholine receptors in bovine adrenal chromaffin cells. Toxicology. 2008; 244:77–85. PMID: 18093714.

32. Ambruosi B, Uranio MF, Sardanelli AM, Pocar P, Martino NA, Paternoster MS, Amati F, Dell'Aquila ME. In vitro acute exposure to DEHP affects oocyte meiotic maturation, energy and oxidative stress parameters in a large animal model. PLoS One. 2011; 6:e27452. PMID: 22076161.

33. Chauvigné F, Plummer S, Lesné L, Cravedi JP, Dejucq-Rainsford N, Fostier A, Jégou B. Mono-(2-ethylhexyl) phthalate directly alters the expression of Leydig cell genes and CYP17 lyase activity in cultured rat fetal testis. PLoS One. 2011; 6:e27172. PMID: 22087261.

34. Kado RT, Baud C. The rise and fall of electrical excitability in the oocyte of Xenopus laevis. J Physiol (Paris). 1981; 77:1113–1117. PMID: 6286961.
35. Gil Z, Magleby KL, Silberberg SD. Membrane-pipette interactions underlie delayed voltage activation of mechanosensitive channels in Xenopus oocytes. Biophys J. 1999; 76:3118–3127. PMID: 10354436.

36. Rettinger J. Novel properties of the depolarization-induced endogenous sodium conductance in the Xenopus laevis oocyte. Pflugers Arch. 1999; 437:917–924. PMID: 10370071.

37. Trexler EB, Bukauskas FF, Bennett MV, Bargiello TA, Verselis VK. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J Gen Physiol. 1999; 113:721–742. PMID: 10228184.
38. Hamill OP, McBride DW Jr. Rapid adaptation of single mechanosensitive channels in Xenopus oocytes. Proc Natl Acad Sci U S A. 1992; 89:7462–7466. PMID: 1380158.

39. Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999; 283:1176–1180. PMID: 10024245.

40. Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009; 458:597–602. PMID: 19340074.
41. Poole A, van Herwijnen P, Weideli H, Thomas MC, Ransbotyn G, Vance C. Review of the toxicology, human exposure and safety assessment for bisphenol A diglycidylether (BADGE). Food Addit Contam. 2004; 21:905–919. PMID: 15666984.

42. Lee IK, Rhee SK. Inhibitory effect of bisphenol A on gap junctional intercellular communication in an epithelial cell line of rat mammary tissue. Arch Pharm Res. 2007; 30:337–343. PMID: 17424940.

Fig. 1
Voltage-dependent gating of Cx46 hemichannel. (A) When holding the oocyte expressing Cx46 hemichannels at -70 mV, currents were near zero level. When stepping to a depolarizing potential at +30 mV for 10 sec, slowly activating outward currents were observed. When stepping back to the initial holding potential at -70 mV, relatively fast inward tail currents were shown. Immediately after, currents were back to zero level. The total recording time was 40 sec. (B) Same pulse protocol used in Fig. 1A was applied to un-injected oocyte. Slowly activating outward and relatively fast inward currents as those of Cx46 cRNA injected oocytes were not observed from the un-injected oocyte. (C) Same pulse protocol used in Fig. 1A was applied to each sweep. One set of records included a total of nine sweeps (3 of each control, drug, and wash phases, respectively) and were obtained from a single oocyte expressing Cx46 hemichannels. Each sweep record was acquired immediately after the perfusion with new bath solution. Most peak currents were quite stable for the entire recording period indicated by a dotted line. The voltage-dependence of Cx46 hemichannels represented by slowly activating outward currents and relatively fast inward currents were observed without any change. Current traces for about 25 seconds before the next sweep were omitted for data illustration.
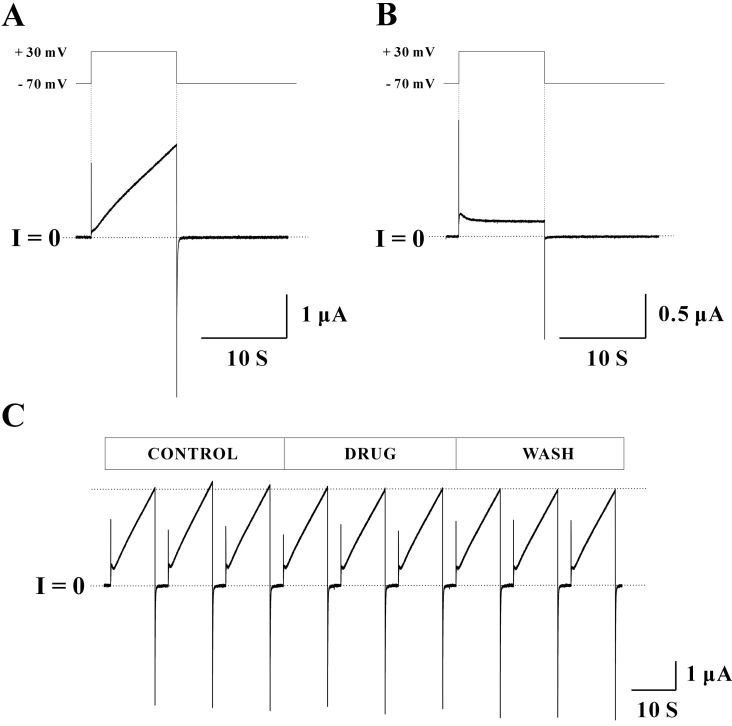
Fig. 2
The effect of BPA (10 nM) on Cx46 hemichannel currents. (A) One set of records obtained from a single oocyte expressing Cx46 hemichannels is shown. The pulse protocol included VH=-70 mV, Vstep=+30 mV for 10 sec, and a total recording time of 40 sec was used to each sweep. In the control phase (initial three sweeps), the average peak current was 4.75±0.48 µA. The average peak current after applying 10 nM BPA (middle three sweeps) was 3.45±0.05 µA, indicating that BPA inhibited Cx46 hemichannel currents to 27.36% of control. Reversible effects of BPA were observed. In the wash phase (last three sweeps) these reduced currents were returned to control level (4.64±0.40 µA). Overall shapes for outward and inward currents were not affected by BPA. Current traces for about 20 seconds before the next sweep were omitted for better illustration. (B) Normalized peak currents of each phase determined by the ratio of average peak currents of the drug (or wash) phase to those of the control phase are shown. Normalized peak currents with BPA treatment were 0.82±0.10 (n=5). This inhibitory effect of BPA (~18% reduction) was statistically significant (*p<0.05). However, normalized peak currents in the wash phase (1.06±0.15, n=5) were not statistically different from that of control, meaning that BPA effect was reversible.
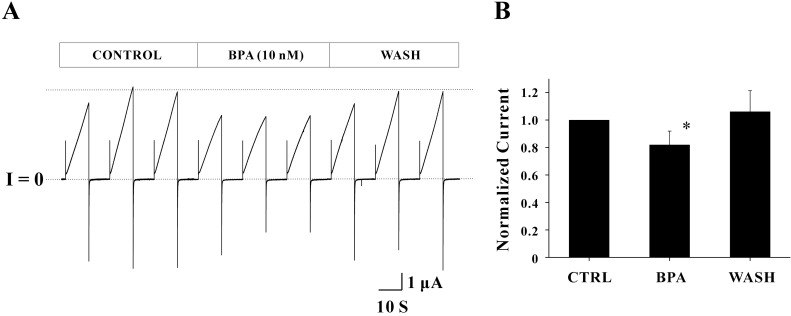
Fig. 3
The effect of OP (1 µM) on Cx46 hemichannel currents. (A) One set of records obtained from a single oocyte expressing Cx46 hemichannels are shown. The pulse protocol included VH=-70 mV, Vstep=+30 mV for 10 sec, and a total duration of 40 sec was used to each sweep. The average peak current in the control phase (1.60±0.13 µA) was reduced to 1.06±0.02 µA by applying 1 µM OP (middle three sweeps). The reduction rate of currents for this particular record was 33.72% of control. The inhibitory effect of OP was reversible by recovering reduced current back to 1.52±0.06 uA. Overall shapes for outward and inward currents were unchanged by OP. Current traces for about 25 seconds before the next sweep were omitted for better illustration. (B) All data obtained from the four independent experiments were analyzed by normalizing peak currents of each drug (or wash) phase to those of the control phase. Normalized peak currents inhibited by OP were 0.72±0.10 (n=4) and this current reduction (~28% of control) was statistically significant (*p<0.05). Inhibitory effect of OP was also reversible (0.99±0.07, n=4).
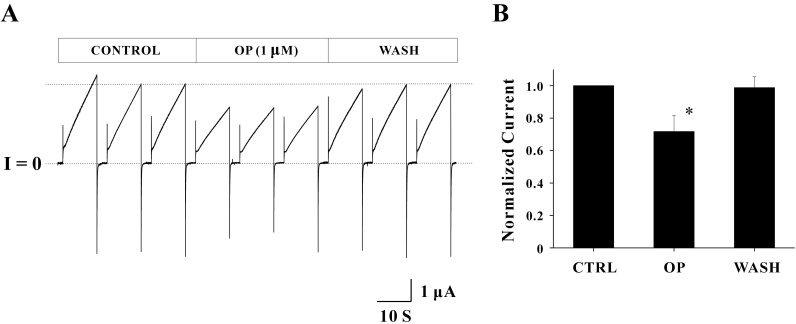
Fig. 4
The effects of other drugs on Cx46 hemichannel currents. (A) Representative current traces obtained from oocytes expressing Cx46 hemichannels with drug treatments are shown. The pulse protocol (VH=-70 mV, Vstep=+30 mV for 10 sec, and a total duration of 40 sec) was used for each sweep. The horizontal thick bars on the top of current traces indicate drug phases (from the top panel, NP, BP, MEHP, and DEHP, respectively). The average peak currents in control phase (initial three current traces of each record) were not changed by drug treatments indicated by dotted lines. Current traces and overall shapes for outward and inward currents of Cx46 hemichannels were not modified by these drugs. None of them appeared to block Cx46 hemichannel currents. Current traces for about 20 seconds before the next sweep were omitted for better illustration. Scale bars indicate 10 sec and 1 µA. (B) Normalized peak currents obtained from the independent experiments are shown. Normalized peak current of each phase was determined by the ratio of average peak currents of the drug (or wash) phase to those of the control phase. Normalized peak currents with drug treatments (0.99±0.05 for NP (n=3), 1.03±0.05 for BP (n=3), 0.98±0.07 for MEHP (n=4), 1.01±0.06 for DEHP (n=3), respectively) were not significantly different from those of control.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download